
Balance Perturbation Leads a Stretching Reflexion on Tibialis
Anterior Muscle
Renata Gonçalves Pinheiro Corrêa
1
, Matheus Lucas Aguiar
1
, Caluê Papcke¹, Eduardo Borba Neves
2
,
Agnelo Denis Vieira
1
and Eduardo Mendonça Scheeren
1
1
Pontifícia Universidade Católica do Paraná, Polytecnic School, Health Tecnology Graduate Program,
Rua Imaculada Conceição 1155, Curitiba, Brazil
2
Universidade Tecnologica Federal do Paraná, Biomedical Engineering Graduate Program, Curitiba, Brazil
Keywords: Postural Balance, Electromyography, Neural Inhibition.
Abstract: Human posture control is a sophisticated process involving the relationship among multiple joints, muscle
groups and environment. The aim of this study is to show how the balance perturbation (in posterior-
anterior direction) leads to a stretching reflexion on the tibialis anterior (TA) muscle. A case study that
involved a male participant with 23 years old. To disturb the participant's balance, it has been employed a
specially plataform designed with dimensions 1.5 m by 1.5 m with movement of 5 mm of amplitude on a
total time of 3 ms along the axis coinciding with participant's anterior-posterior axis. Soleus (SO) and TA
electromyography signal (EMG) has been recorded. Perturbation in the equilibrium was delivered in the
posterior-anterior direction. The first event observed was the pre-activation of the TA muscle that leads a
reduction in the SO muscle activation, due the stretch reflex at TA.
1 INTRODUCTION
Human posture control is a sophisticated process
involving the relationship among multiple joints,
muscle groups and environment (Horak, 2006).
Theoretically, the passive muscle stiffness can
ensure postural control in upright position under
static conditions (Neves et al., 2013). However in
practice, it is necessary coordinated muscle activity
to ensure balance in daily activities (de Medeiros et
al., 2010). The two main functional goals of postural
control are: postural orientation and balance.
Postural balance involves movement strategies to
stabilize the center of mass within the base of
support due to stability disturbances externally
caused (Gage, 2004; Horak, 2006; Robinson, 2011).
Static balance is guaranteed when the sum of all
external forces and all external torques acting on the
body equals zero, however, under the mechanical
point of view, the body is never in a condition of
perfect balance, as the sum of forces and torques
acting on it are only momentarily zero. Thus, Duarte
and Freitas (2010) propose that the human body is in
constant unbalance and in an endless quest for
balance.
Maintenance of body balance in the environment is
determined by central systems and peripheral
structures responsible for motor execution, whose
functioning depends on the integration of
information from the sensory structures of the
proprioceptive, vestibular and visual systems. These
sensory structures respond in complex and different
ways for each perturbation on the body (Melzer,
Benjuya and Kaplanski, 2004).
For balance control to occur in a harmonious
way the sensory system is used to obtain continuous
and integrated information on position and trajectory
of the body, allowing the issuance of motor
responses arranged to be performed by the
osteomioarticular effector system (Robinson, 2011).
There are three basic strategies for correcting
body balance as responses to postural perturbations:
(a) the strategy of the ankle, where the body moves
as a relatively rigid mass about the ankle, (b) hip
strategy, used when the center of gravity (CG)
moves quickly but with a relatively small
displacement or when the support base is narrow or
unstable and (c) a step strategy used when the GC is
scrolled beyond the limits of stability (Horak et al.,
1989).
324
Gonçalves Pinheiro Corrêa R., Lucas Aguiar M., Papcke C., Borba Neves E., Denis Vieira A. and Mendonça Scheeren E..
Balance Perturbation Leads a Stretching Reflexion on Tibialis Anterior Muscle.
DOI: 10.5220/0005283103240328
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2015), pages 324-328
ISBN: 978-989-758-069-7
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)

Between motor responses, are the stretch reflex and
proprioceptive reflex. These reflexes are involuntary
responses to external stimuli in different movements,
automated or forecasting.
Corporal movements to balance recovery can
trigger reactive fast motor response that leads a
muscle pre-activation, sudden stretch and inhibition
of antagonist muscles. One of these reactive a motor
response is the reciprocal inhibition, which is an
important neurophysiological phenomenon leading
to better coordination of movements and efficiency
(Robinovitch and Murnaghan, 2013). According to
Hortobagyi et al. (2006), the reciprocal inhibition
may decrease with age and contribute to poor
performance of movements in older people, because
it is an important tool in neurophysiological motor
coordination.
Through the disturbance of equilibrium occurs
subsequent order: (a) muscle pre-activation; (b)
sudden muscle stretch; (c) inhibition of the
antagonist muscles. The aim of this study is to show
how the balance perturbation (in posterior-anterior
direction) leads to a stretching reflexion on the
tibialis anterior (TA) muscle.
2 METHOD
A case study was conducted with a 23 years old
male participant, 67kg of body mass and 1.79 m tall,
to show how the balance perturbation leads to a
stretching reflexion on the tibialis anterior muscle.
This study has been approved by Human Research
Ethics Committee of Pontifícia Universidade
Católica do Paraná (PUCPR) under register number
620.735.
2.1 Experimental Protocol
The experimental protocol comprises the
participant's balance perturbation with concurrent
record of balance maintenance muscular action. The
participant has been told to stand still on orthostatic
position on a movable platform keeping 17cm of
feet aperture and 14° of ankle abduction, facing
forward the researcher. Initially the movable
platform has remained still while the participant was
advised that it would start moving. After some
random time, suddenly and without participant's
warning the platform has performed an alternative
movement of 5mm of amplitude on a total time of
3ms on the anterior-posterior direction. Along this
time the (TA) and Soleus (SO) muscular action has
been recorded through a data acquisition system fed
by electromyography signal (EMG). The participant
has kept his eyes open along the experiment. The
platform has started moving from participant's
posterior to anterior side, a perturbation similar as
pulling a carpet intending to cause the participant to
fall sitting. Figure 1 shows the research's participant
standing still on the movable platform.
2.2 Movable Platform
In order to disturb participant's balance it has been
employed a specially designed 1.5m by 1.5 m
platform which is allowed to move up to 0.9 m along
both horizontal orthogonal axes. In this particular
protocol, the platform has been driven by a linear
pneumatic actuator performing an alternative
movement of 5 mm of amplitude on a total time of 3
ms along the axis coinciding with participant's
anterior-posterior axis. Displacement has been
measured with a potentiometric linear transducer.
2.3 Electromyography
Muscular activation has been measured employing a
four channel surface electromyography system.
Site's skin preparation and placement of electrodes
have been in agreement with that stated by SENIAM
project (SENIAM, 2014). Electrodes's site
preparation comprised body hair shaving followed
by skin abrasion employing cotton and alcohol 70°.
It has been employed self-adhesive surface
electrodes in a bipolar configuration (Ag/AgCl
2,2cm) positioned at 1/3 on the line between the
tip of the fibula and the tip of the medial malleolus
in the direction of the line between the tip of the
fibula and the tip of the medial malleolus for TA and
at 2/3 of the line between the medial condyles of the
femur to the medial malleolus in the direction of the
line between the medial condyles to the medial
malleolus for SO. Reference's electrode was placed
around the ankle at the anterior face of Tibia.
2.4 Data Acquisition and Processing
Data acquisition has been performed employing a
National Instruments board, model NI-USB-6009,
and a specially designed software developed on
LabView with a sampling rate of 2KHz with input
analog channels on a differential configuration
resulting in a 14 bits resolution.
Data processing has been performed on Matlab,
comprising the following steps:
i) EMG RMS envelop determination;
ii) Muscular recruitment threshold determination;
BalancePerturbationLeadsaStretchingReflexiononTibialisAnteriorMuscle
325
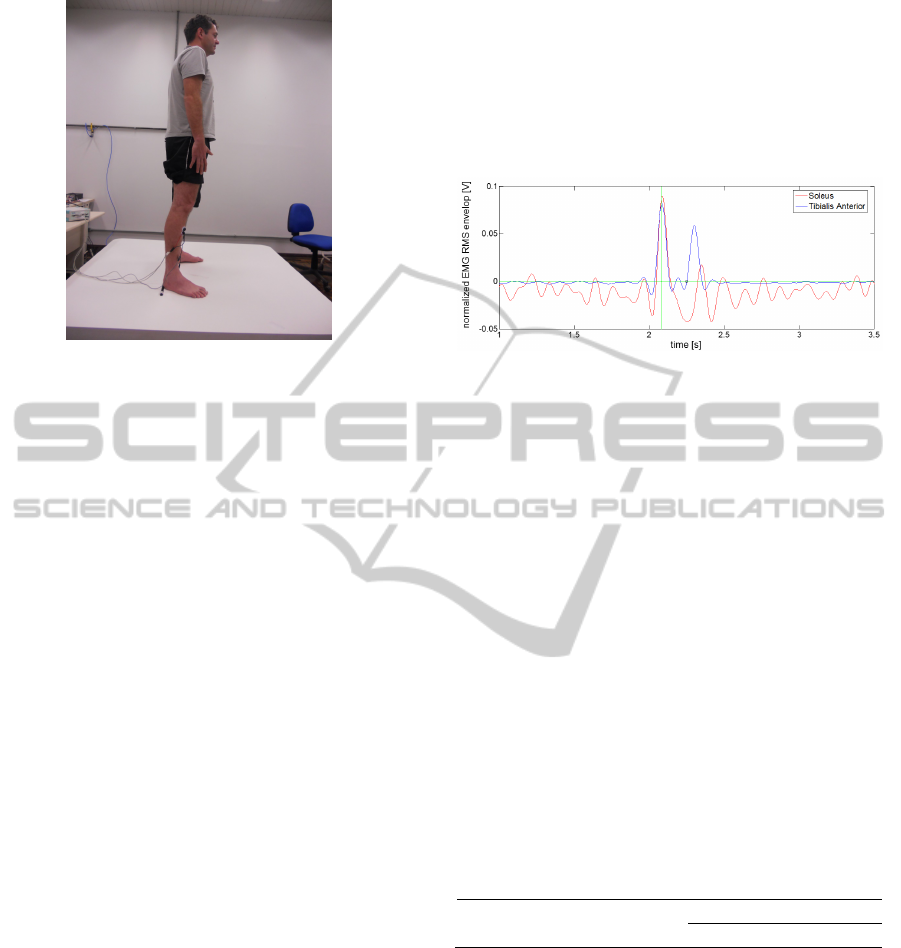
Figure 1: Research's participant standing still on the
movable platform with EMG electrodes on TA and SO
muscles.
iii) Start of muscular recruitment determination;
iv) Time delay between start of balance's
perturbation and start of muscular recruitment
determination;
v) EMG RMS envelop normalization;
Items (i) to (v) have been performed
independently and sequentially from TA to SO
muscles.
EMG RMS envelop determination has been
performed employing function "filtfilt" of Matlab.
This function's input arguments where numerator
and denominator of a 5th order Butter-Worth low-
pass filter with a 20Hz cut-off frequency and 2 kHz
sample frequency obtained by function "butter" of
Matlab. The third input argument of function
"filtfilt" was the absolute value of corresponding
EMG.
A 1s lasting window of the EMG RMS envelop
preceding perturbation's start has been selected on a
monitor's computer plot by visual inspection. The
mean value and the standard deviation of EMG
RMS envelop into this window have been obtained.
Muscular recruitment threshold has been obtained
by the sum of 100% of mean value and 150% of
standard deviation.
Based on visual inspection of a plot, the
researcher has been allowed to select the instant of
time that the value of EMG RMS envelop has
become greater than the muscular recruitment
threshold. This has been considered the start of
muscular recruitment. Time of start of balance's
perturbation was obtained based on displacement's
transducer signal. Subtracting these two time values
has resulted on the time delay between start of
balance's perturbation and start of muscular
recruitment.
EMG RMS envelop normalization has been
performed by subtracting the muscular recruitment
threshold from corresponding EMG RMS envelop
value. Doing so, one may consider that both muscles
have a null normalized muscular recruitment
threshold.
Figure 2: Normalized EMG RMS envelop of TA (blue)
and SO (red); selected start of TA muscular recruitment
(small blue circle); normalized muscular recruitment
threshold (green horizontal line); start of balance's
perturbation (green vertical line).
3 RESULTS
Through the identification of contraction thresholds
of TA and SO muscles and using the normalized
EMG RMS envelop it has been possible to
determine temporal behavior of muscle contraction
(Figure 2). This behavior corresponds to the
situation in which the participant was with eyes open
and with early motion of the platform on the
posterior-anterior direction. The time delay between
start of balance's perturbation and start of muscular
recruitment contraction (muscle contraction delay)
of TA and SO muscles are presented at Table 1.
Table 1: Muscle contraction delay time for TA and SO
muscles.
TA (ms) SO (ms)
Muscle contraction delay 0.0164 0.0462
As the values shown at Table 1, the TA and SO
muscles contracted at different times. This was
expected due to their antagonistic action. Whereas
the balance disorder had higher-anterior direction
and the first muscle to contract was the TA (Table 1
and Figure 2), we believe that the ankle joint,
initially held a plantar flexion. In this movement, the
TA muscle due to its dorsi-flexor action is stretched
and rapidly contracts to maintain the balance.
As the SO muscle performs plantar flexion, and
joint action of the ankle resulting from the balance
disturbance is the same, it was activated later (Table
BIOSIGNALS2015-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
326

1) to the action of the TA muscle for adjusting joint
position after dorsiflexion trend held by TA.
4 DISCUSSION
Despite that the research's participant didn´t know
the exact time of the perturbation, he knew that the
disturbance would happen at any time. In this
situation, Stokes et al. (2000) suggest that pre-
activation occurs in the muscles of the participant
who is subjected to a disturbance of equilibrium,
which is also associated with an increased sensitivity
of the muscle spindle, causing changes in length and
increasing the probability of responding to a sudden
muscle disorder.
Cort et al. (2013) analyzed the contributions of
the muscles of the spine due to balance's disorders,
they concluded that the participants also performed
muscle contractions prior to perturbation. This
enhances the performance of the neuromuscular
system to responses to sudden balance's disorders,
increasing the sensitivity of the receptors responsible
for detecting motion (Cort et al., 2013).
Figure 2 shows that there is an increase in SO
muscle tone prior to perturbation. However, after the
disturbance, the EMG RMS envelop signal
undergoes a sudden attenuation compared to the pre-
perturbation. We believe that this reduction is
associated with transmission's inhibition of the
action potential to the muscle.
Crone et al. (1994), for example, conducted
physical training aimed at increasing the response of
reciprocal inhibition in healthy individuals and in
individuals with musculoskeletal disorders (Geertsen
et al., 2008).
This phenomenon can be explained by the
occurrence of the stretch reflex in the TA muscle
that has the effect of reciprocal inhibition of the
antagonist muscle group to its action.
In a study by Robinovitch and Murnaghan
(2013), using a linear motor connected to a mobile
platform to promote a controlled perturbation, 14
young women were evaluated in three conditions
(forward swing, back swing and static). The results
suggest that the amount of latency (defined as the
time between the onset of the disturbance and the
onset of muscle contraction) in all seven analyzed
muscles (Erector Right Column, Rectus Abdominis,
Soleus, Gastrocnemius, Tibialis Anterior, Rectus
Femoris and Biceps Femoris) seemed to occur
earlier when participants performed a static position.
The authors also concluded that the action of the use
muscles for recovery of the balance are adapted
depending on the nature of the disturbance and the
requested task.
Regarding aging, the work of Piirainen et al.
(2013) monitored EMG signal of muscles SO, TA
and Gastrocnemius of 9 young adults and 10 older
adults in the recovery of postural equilibrium after a
disturbance in the anterior-posterior and posterior-
anterior directions. The authors noted that, due to
aging a decrease in postural control occurs. The
action of SO and TA muscles may not be functional
for maintaining equilibrium in the face of
perturbations in different directions of evaluated
perturbations, indicating a distinct global motor
strategy to balance recovery, and yet, with activation
of antagonistic muscle groups for different
directions of perturbations.
5 CONCLUSIONS
It can be concluded that in the situation of balance
perturbation in posterior-anterior direction, the TA
muscle contracts before the SO, and the SO has pre-
activation, which is inhibited due to the stretch
reflex that occurs in the TA muscle.
REFERENCES
Cort, J. A., Dickey, J. P. & Potvin, J. R. 2013. Trunk
muscle contributions of to L4-5 joint rotational
stiffness following sudden trunk lateral bend
perturbations. J Electromyogr Kinesiol, 23, 1334-42.
De Medeiros, V. M. L., De Lima, F. M. R. & Di Pace, A.
M. 2010. Equilíbrio, controle postural e suas
alterações no idoso.
Duarte, M. & Freitas, S. 2010. Revisão sobre
posturografia baseada em plataforma de força para
avaliação do equilíbrio. Rev bras fisioter, 14, 183-92.
Geertsen, S. S., Lundbye-Jensen, J., Nielsen, J. B., 2008.
Increased central facilitation of antagonist reciprocal
inhibition at the onset of dorsiflexion following
explosive strength training. Journal of appl physiol;
105 (3): 915-22.
Horak, F. B. 2006. Postural orientation and equilibrium:
what do we need to know about neural control of
balance to prevent falls? Age Ageing, 35 Suppl 2, ii7-
ii11.
Horak, F. B., Shupert, C. L. & Mirka, A. 1989.
Components of postural dyscontrol in the elderly: a
review. Neurobiology of aging, 10, 727-738.
Linford, C. W., Hopkins, J. T., Schulthies, S. S., Freland,
B., Draper, D. O. & Hunter, I. 2006. Effects of
neuromuscular training on the reaction time and
electromechanical delay of the peroneus longus
BalancePerturbationLeadsaStretchingReflexiononTibialisAnteriorMuscle
327

muscle. Archives of physical medicine and
rehabilitation, 87, 395-401.
Melzer, I., Benjuya, N., Kaplanski, J., 2004. Postural
stability in the elderly: a comparison between fallers
and non-fallers. Age Ageing, 33 (6): 602-7.
Murnaghan, C. D. & Robinovitch, S. N., 2013. The effects
of initial movement dynamics on human responses to
postural perturbations. Human movement science, 32,
857-865.
Neves, E. B., Krueger, E., Stéphani De Pol, M. C., De
Oliveira, N., Szinke, A. F. & De Oliveira Rosário, M.
2013. Benefícios da Terapia Neuromotora Intensiva
(TNMI) para o Controle do Tronco de Crianças com
Paralisia Cerebral. Rev Neurocienc, 21, 549-555.
Page, P., 2006. Sensorimotor training: A “global”
approach for balance training. Journal of Bodywork
and Movement Therapies, 10, 77-84.
Piirainen, J. M., Linnamo, V., Cronin N. J., Avela J.,
2013. Age-related neuromuscular function and
dynamic balance control during slow and fast balance
perturbations. J Neurophysiology, 110:2557-2562.
Robinson, C. A., Shumway-Cook, A., Ciol, M. A., Kartin,
D., 2011. Participation in community walking
following stroke: subjective versus objective measures
and the impact of personal factors. Phys ther, 91:1865-
1876.
SENIAM, 2014. Surface ElectroMyoGraphy for the Non-
Invasive Assessment of Muscles project.
(http://www.seniam.org).
William H. Gage, W. H., Winter, D. A., Frank, J. S.,
Adkin, A. L., 2004. Kinematic and kinetic validity of
the inverted pendulum model in quiet standing. Gait $
Posture, 19 (2): 124–132.
BIOSIGNALS2015-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
328
