
The Transparent Representation of Medical Decision Structures
Based on the Example of Breast Cancer Treatment
D. Andrzejewski
1
, L. Tetzlaff
1
, J. DeBoer
1
,
E. Beck
1
and N. Haeusler
2
1
University of Applied Computer Science Brandenburg, Department Informatics and Media,
Magdeburger Straße 50, Brandenburg, Germany
2
Städtisches Klinikum Brandenburg GmbH, Geburtshilfe und Frauenheilkunde, Brandenburg, Germany
Keywords: Medical Decision Structures, Representation, Decision Support, DMN.
Abstract: Choosing the appropriate treatment for patients have a direct influence on each patient`s future. A doctor´s
expertise, the patient´s preferences, and the current medical research have a highly influence on the choice of
the treatment. Doctors shall be aware of their own decision patterns, the most influenced factors and the
relevant literature by choosing the optimal patients treatments. By considering quality management and
certifications, transparent representations of internal processes with simple decision-making notes are
required. In support of the hypothesis, a decision analysis was conducted based on the S3 guideline for
diagnosis, treatment and follow up care of breast cancer. A notation is required, which combines the process
modeling and the representation of (medical) decisions.
1 INTRODUCTION
A transparent representation of processes is a useful
gadget for physicians and other medical
professionals, especially considering the
requirements for quality management and
certifications. Medical decisions are generally based
on physicians’ expertise, patients’ preferences and the
relevant recommendations based on the best available
medical evidence. However, most decision structures
are only “present in the medical experts head”, which
means, they are not at hand in a standardized,
structured and thus “non-expert-understandable”
form. The representation of decisions has been
modelled in the past with The Decision Model (von
Halle, Goldberg, 2009). In our partner clinic
“Städtisches Klinikum Brandenburg GmbH” the
representation of processes and decisions will be
modelled mostly with Microsoft Visio. This
representation has the main problem that the clarity
and comprehension suffer. The Object Management
Group developed the Decision Model Notation
(DMN) as possibility to represent decisions
transparently in an easy way of understanding and
handling (Object Management Group, 2014).
Therefore this paper tries to answer the question if a
representation of medical decisions with DMN is
possible. Also the supporting data are required for a
transparent decision representation.
2 MATERIAL AND METHODS
2.1 Decision Model Notation (DMN)
The Object Management Group (OMG) published in
January 2014 the first Beta version of Decision Model
Notation (DMN). The primary goal of this notation is
design readable, understandable and transparent
decision models for every kind of user.
DMN is a new standard to combine business
decision design and decision implementation. Those
decisions need to be analysed and represented (Object
Management Group, 2014). The three aspects of
modelling are as followed: Business Processes (e.g.
modelled in BPMN), Decision Requirements
Diagram (modelled in DMN) and the Decision Logic.
A Business Process Model is e.g. a transparent
representation of an internal procedure of a company.
A Decision Requirements Diagram is a diagram to
represent the decision with different elements (like
knowledge elements or input data elements) and the
Decision Logic represents the analysed rules in form
617
Andrzejewski D., Tetzlaff L., DeBoer J., Beck E. and Haeusler N..
The Transparent Representation of Medical Decision Structures Based on the Example of Breast Cancer Treatment.
DOI: 10.5220/0005283306170621
In Proceedings of the International Conference on Health Informatics (HEALTHINF-2015), pages 617-621
ISBN: 978-989-758-068-0
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)
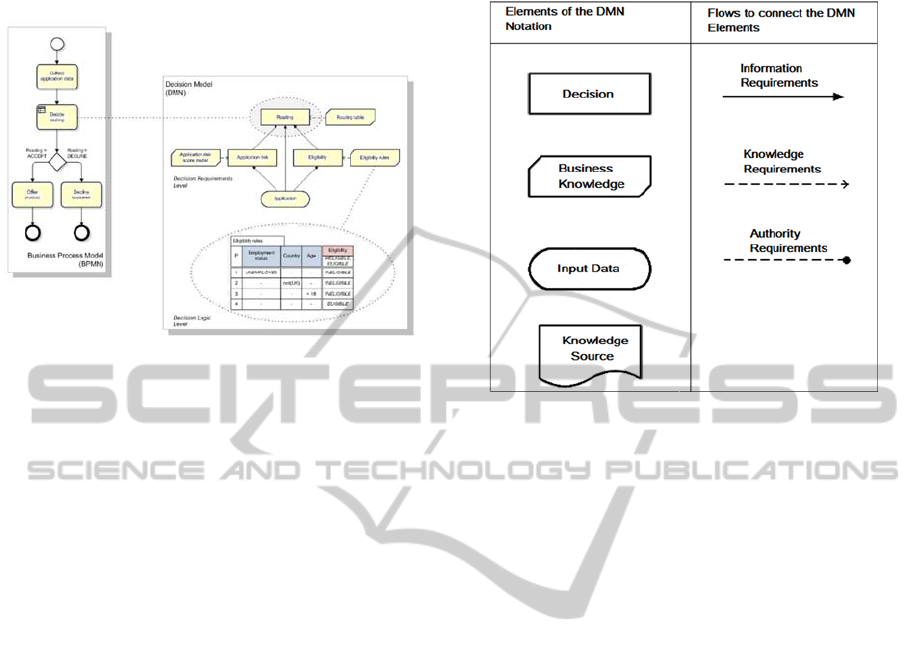
of a decision table. Figure 1 shows the three aspects
of modelling.
Figure 1: Three aspects of modelling (OMG, 2014).
It is very important to understand that there are two
different standards for modelling decisions which are
defined as followed:
Business process modelling, using e.g.
Business Process Model and Notation
(BPMN).
Decision logic, which defines business rules by
the way of decision tables and can be used for
individual decisions.
In BPMN it is possible to define business rules via the
BPMN Business Rule task. This task is the link to the
DMN notation. At this time there is currently no tool
which combines the modelling with BPMN and
DMN, except the Signavio Process Editor. The
intension of DMN is to combine business process
models and decisions logics (Object Management
Group, 2014). The following three aspects of
modelling are relevant:
Business process models are defining tasks in
procedures, which may include decisions to be
reached.
Decision requirements diagrams (DRDs)
specify the kind of decision to be made and the
information required. DRDs consist of
different elements: the specified decision,
required business knowledge, an Input-Data-
Model, the knowledge source and the different
connectors to model the flows which combine
these elements (information, knowledge and
authority requirements).
Decision logics should represent the necessary
decisions in such detailed manner that valid
decisions in an automated fashion can be made
details for validate decisions and automate the
decisions also.
Figure 2 visualizes the elements of DMN:
Figure 2: Elements of the DMN (OMG, 2014).
In summery a decision structure can be represented
with a Decision Requirements Diagram (DRD) and a
Decision Logic. Those can be combined also with a
business process model in the relevant task.
2.2 Breast Cancer – Tumour Board
and Relevant Decisions
Breast cancer is the most common malignant
neoplasia in females in Germany, accounted for
approximately 70.000 new cases per year. After
confirming the diagnosis by histopathological
examination, the attending physician will inform the
patient about the further steps, which follow after
diagnosis. In some cases prior to this, the patient´s
case will be discussed in a pre-operative tumour
conference. However, according to the S3-guideline
(Kreienberg et.al, 2012) all cases will be discussed in
the postoperative tumour board. The attending
physician presents the individual case to the members
of the board and recommends the patient-individual
treatment choices. Then the board discusses the
different options and finally decides which
therapeutic regiment is the most promising, based on
the recommendations of the S3-guideline. This
decision will take place in form of a formal
consensus. The decision is then formally documented
and forms the basis for the further treatment. In figure
3 this process is visualized as BPMN model.
But which parameters are relevant for such a
decision? According to Kreienberg et.al the patient´s
age, her menopausal status, the expression of estogen
and/or progesterone receptor and HER2/neu status of
HEALTHINF2015-InternationalConferenceonHealthInformatics
618
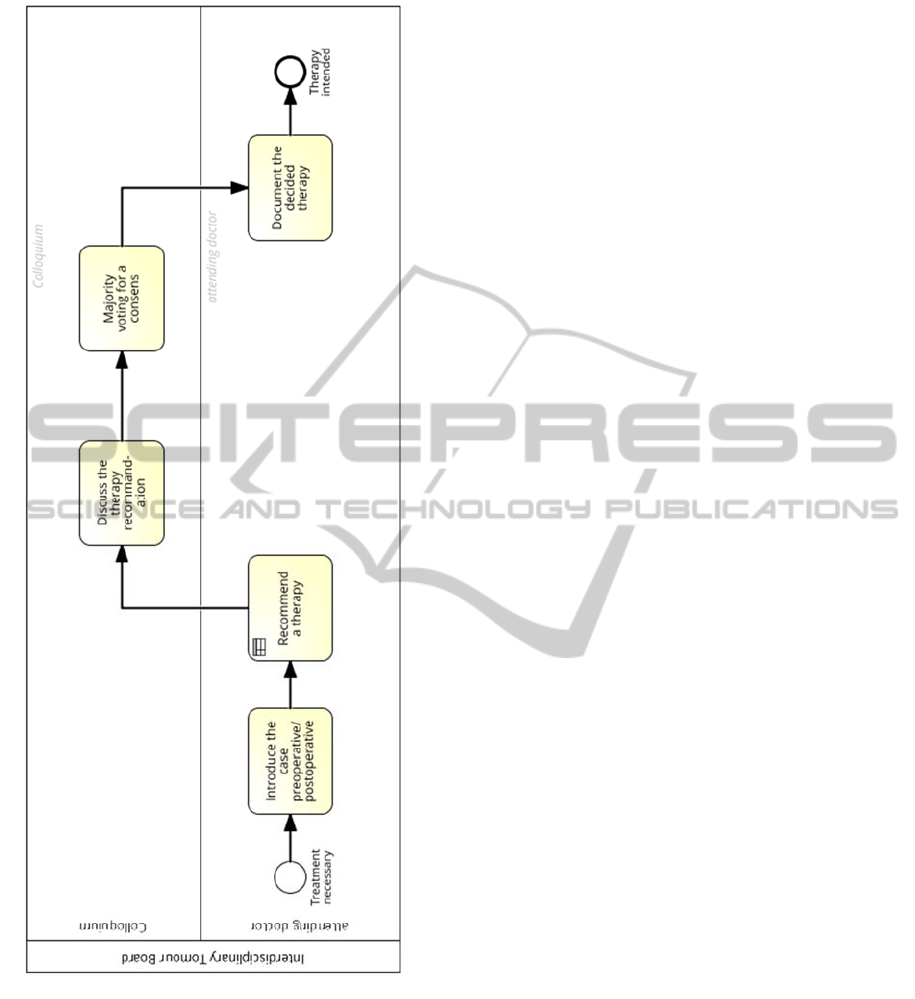
Figure 3: Tumour board modelled in BPMN.
the tumour, Grading and the tumour and lymph node
classification are required.
For this paper the systemic treatment of the patient
will only be considered – consisting of either
chemotherapy or endocrine therapy, a combination of
both modalities, and/or the anti HER-2/neu (Human
epidermal growth factor receptor 2) treatment. Based
on the above listed tumour and patient related
parameters according to Kreienberg R. et.al: the
following rules concerning therapeutic decisions can
be derived:
The patient`s menopausal status is required for
the choice of the endocrine therapy
A primary (neoadjuvant) chemotherapy should
be recommended in cases of triple negative
breast cancer (i.e. estrogen receptor and
progesterone receptor and HER-2/neu negative
tumours)
A primary (neoadjuvant) chemotherapy is
mandatory in cases of inflammatory breast
cencer or advanced tumour stages (T4)
A primary (neoadjuvant) chemotherapy could
be recommended in cases with a positive HER-
2/neu status and/or a pathological tumour size
of >= pT1c and a high grading (G2 or G3, or a
positive nodal status and/or a positive
HER2/neu status and/or a positive or negative
hormone receptor (HR) status.
In cases of positive HR status and a grading of
G2 further tests like uPA/PAI1 or gene
expression signatures can be performed
Endocrine treatment (ET) is required in cases
of positive HR-status
Anti HER-2/neu antibody treatment is required
in cases with positive HER2/neu status (which
is always applied in combination or sequential
to a chemotherapy regimen).
Tumour related parameters are important to describe
the biology and pathology of the tumour. Gathered
with patient related parameters, as the patient´s age
and menopausal status, they are required to model
valid DRDs. These parameters are the basement of
the breast cancer treatment and they are represented
as Input-Data-Model in a DRD. The most relevant
knowledge source is the S3 guideline, published by
the German Cancer Society. The current statements
of the guideline however may be modified by other
relevant literature (Kreienberg R., 2012, Woecke A.,
2010, Wolters R, 2011).
3 RESULTS
The results are represented in two major sections –
the graphical representation of the treatment
decisions for breast cancer modelled in DMN and a
representation of the deduced rules in a decision table.
For a representation of the treatment in DMN three
elements are required: decision element, knowledge
source and Input-Data-element. The decision element
will be labelled with the name “(systemic) therapy
TheTransparentRepresentationofMedicalDecisionStructuresBasedontheExampleofBreastCancerTreatment
619
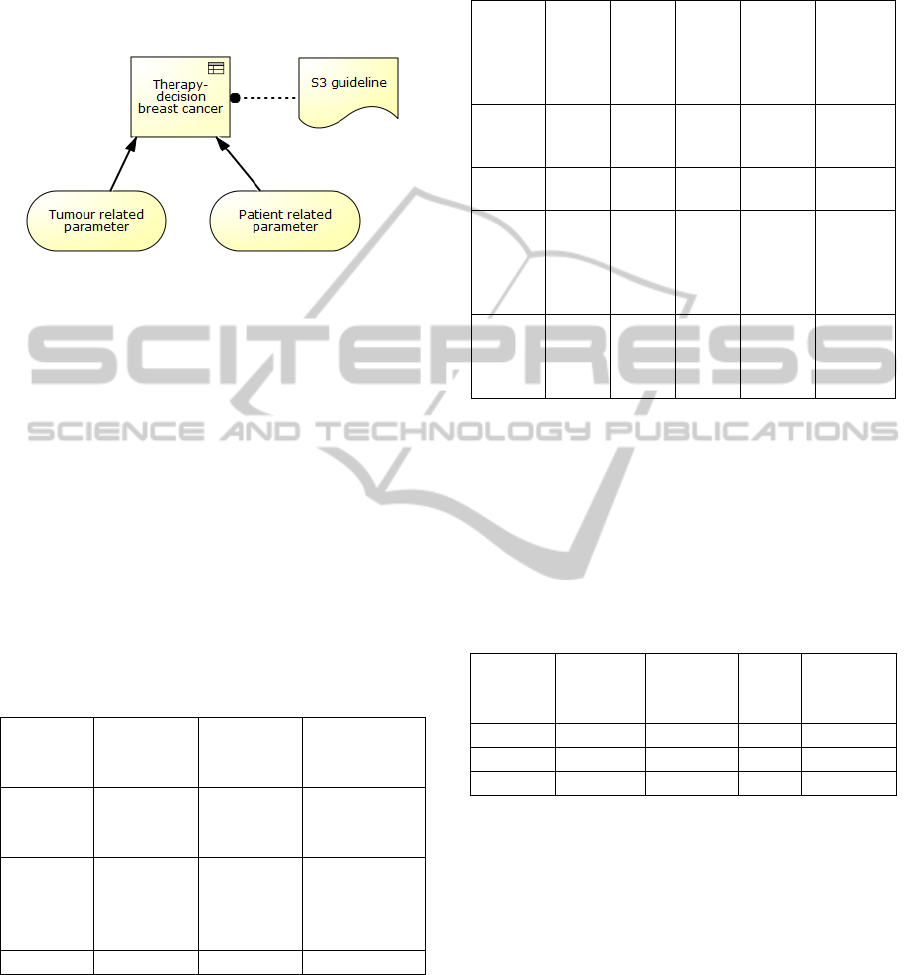
decision for breast cancer”, the two Input-Data-
elements will be labelled with “Tumour and Patient
related parameters” and the knowledge source will be
labelled with “S3 guideline”. The following figure 4
shows the treatment of breast cancer in DMN.
Figure 4: therapeutic decision model of breast cancer
(DMN).
A draft of the decision logic is shown in table 1.
Besides rather simples rules like: “If the patient’s
tumour expresses HER-2/neu, then an anti HER-
2/neu antibody treatment is required” and “If an anti-
HER-2/neu treatment is indicated this has to be
applied simultaneously or sequentially to
chemotherapy”, there are a number of more or less
complex rules. The example for the easy rules
extending the decisions elements to: hormone
receptor status (positive or negative), HER2/neu
status (positive or, negative), Grading (G2, G3) and
therapy (chemo- and anti HER2 therapy (CT+T),
chemo-, anti HER2 and hormone therapy
(CT+T+ET)).
Table 1: example for easy rules.
ER/PgR
status
HER2/
neu
status
Grading Therapy
negative positive G3 chemo- and
anti HER2
therapy
positive positive G2 chemo-, anti
HER2 and
hormone
therapy
… … … …
Examples of more complex rules are e.g. those, based
on the current recommendation of the St. Gallen
consensus meeting (Goldhirsch, 2011, Kreienberg
2012) as shown in table 2.
In cases of an intermediate grading (G2) and a
positive HR-status the decision rules are modified by
introducing the concepts of luminal A and B like
tumors. Luminal B-like tumors are defined either by
Table 2: Risk and therapy decision on breast cancer
subtypes based on (Goldhirsch, 2011).
Sub-
type
Lumi
-nal
A
like
Lumi
-nal
B like
Lumi
-nal
B like
Non
lumina
l
Triple
negativ
e
ER/
PgR
+ + + - -
HER2
- - + + -
Ki-67
Low
<
14%/
G1
High
>
14%/
G3
n/a
n/a
n/a
therap
y
ET
CT +
ET
CT +
T +
ET
CT +
T
CT
ET: Endocrine Therapy, CT: Chemotherapy, T: Trastuzumab
a positive HER-2/neu status or are Ki-67 proliferation
index of > 14%. In cases of luminal A like tumors an
endocrine regimen is sufficient, in cases of luminal B
like tumors a combination of endocrine and
chemotherapy is required. Table 3 transforms the
criteria of the St. Gallen consensus in a clear cut
decision table.
Table 3: St. Gallen Consensus meeting – decision table.
ER/
PgR
status
HER2/
neu
Status
Grading Ki67 Therapy
positive negative G2 high CT + ET
positive negative G2 low ET
… … … … …
The example tables can be modelled as a decision
table in DMN as decision logic. This DMN table
representation is not considered in this paper.
4 CONCLUSIONS
A mandatory regarding the increasing complexity of
clinical decisions as well as patient self-
determination. This aim in our view can be achieved
by modelling decision requirements diagrams using
the Decision Management Notation. The
representation of business rules is likewise achievable
by means of defining a decision logic- therefore the
decision rules must be analysed in the first instance
HEALTHINF2015-InternationalConferenceonHealthInformatics
620

and then represented in DMN. DMN results from a
combination of Business Process Management and
Decision Management (OMG, 2014). Business
Process Management is required for the transparent
representation of business processes and procedures
– the Business Process Model and Notation (BPMN)
being one of the possibilities for modelling the
processes. For the analysis of business rules a
structured approach is required. According to Taylor
there are four principles to be kept in mind when
analysing decision rules (Taylor, 2011).
1. Model the decisions, which are in the experts
head
2. Be flexible, transparent and agile for changes
3. Be predictable
4. Combine all three rules and continue with the
first task
The recommendations are proven as good approach
for the analysis of business rules. However, to our
knowledge, there are no recent papers concerning this
approach in medicine. Further work is now centred on
the validation of our so far analysed and modelled
decision rules. Physicians and other medical staff can
benefit from transparent representation of the
processes and the modelled decision rules.
REFERENCES
Kreienberg R. et.al, (2012) Interdisziplinäre S3 Leitlinie zur
Diagnostik, Therapie und Nachsorge des
Mammakarzinoms.
Goldhirsch et al, (2011) Strategies for subtypes – dealing
with the diversity of breast cancer: highlights of the St
Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011.
von Halle B und Goldberg L, (2009) The Decision Model,
1
st
Edition, 2009.
Woecke A. et al, (2010) Impact of Guideline Conformity on
Breast Cancer Therapy: Result of a 13-Year
Retrospective Cohort (2010).
Wolters R. et al, (2011) Auswirkungen leitlinienkonformer
Therapie auf das Überleben von Patientinnen mit
primärem Mammakarzinom – Ergebnisse einer
retrospektiven Kohortenstudie (2011).
Taylor J., (2011) Decision Management Systems, 1
st
Edition, 2011.
Object Management Group (OMG), Decision Model
Notation (DMN) Beta Version 1.0, 2014.
TheTransparentRepresentationofMedicalDecisionStructuresBasedontheExampleofBreastCancerTreatment
621
