
Estimation of Postoperative Knee Flexion at Initial Contact of Cerebral
Palsy Children using Neural Networks
Omar A. Galarraga C.
1,2
, Vincent Vigneron
2
, Bernadette Dorizzi
3
, Néjib Khouri
1,4
and Eric Desailly
1
1
UNAM, Pôle Recherche & Innovation, Fondation Ellen Poidatz, 1 rue Ellen Poidatz, Saint-Fargeau-Ponthierry, France
2
IBISC EA 4526, Université d’Evry Val d’Essonne, 40 rue du Pelvoux, Courcouronnes, France
3
Institut Mines-Télécom, Télécom SudParis, UMR 5157 SAMOVAR, 9 rue Charles Fourier, Evry, France
4
Hôpital Universitaire Necker-Enfants malades, 149 rue de Sèvres, Paris, France
Keywords:
Clinical Gait Analysis, Nonlinear Data Fitting, Neural Networks, Cerebral Palsy, Biomechanics.
Abstract:
Cerebral Palsy (CP) affects walking and often produces excessive knee flexion at initial contact (KFIC). Ham-
string lengthening surgery (HL) is applied to decrease KFIC. The objective of this work is to design a simulator
of the effect of HL on KFIC that could be used as a decision-making tool. The postoperative KFIC is esti-
mated given the preoperative gait, physical examination and the type of surgery. Nonlinear data fitting is
performed by feedforward neural networks. The mean regression error on test is 9.25
◦
and 63.21% of subjects
are estimated within an error range of 10
◦
. The simulator is able to give good estimations independently of
the preoperative gait parameters and the type of surgery. This system predicts the outcomes of orthopaedic
surgery on CP children with real gait parameters, and not with qualitative characteristics.
1 INTRODUCTION
Cerebral Palsy (CP) is an umbrella term that refers
to a group of neurological disorders from brain dam-
age that affect human movement, balance and posture.
These disorders frequently entail muscle and bone de-
formities. Two typical CP gait troubles are crouch
gait and equinus gait (Gage et al., 2009): crouch gait
is principally characterized by excessive knee flexion
during walking, while equinus gait refers to ground
contact first done by the toes instead of the heel.
In order to lessen these pathological gait patterns,
orthopaedic surgery is usually performed on CP pa-
tients. Multiple bone and soft tissue deformities can
be corrected during a single-event multilevel surgery
(SEMLS) which combines several surgical gestures
according to the functional objective, the technique
applied, the body parts that are affected, etc. For in-
stance, the most common treatment for crouch gait
is hamstring lengthening. Its purpose is to decrease
knee flexion at ground contact by increasing ham-
strings length surgically. This surgery has reportedly
given good results (Ma et al., 2006), however its in-
dication is not always straightforward. First because
this surgery may have side effects on pelvic tilt. Sec-
ond because at this time there is currently no method
or simulation tool, other than the surgeon experience,
that is able to predict hamstring lengthening effect on
knee flexion at initial contact. Physical examination
and clinical gait analysis (CGA) (Gage et al., 2009)
are performed on patients to improve diagnosis and
assert suitable treatments. Specifically for the first
point, most of the useful results are related to muscu-
loskeletal simulations (Arnold et al., 2006; Desailly,
2008; Sebsadji et al., 2012).
On the other hand, simulation studies for predict-
ing effect of treatment on CP gait are rare, e.g. on
equinus gait (Armand et al., 2007) or on effect of rec-
tus femoris transfer surgery (Reinbolt et al., 2009). In
both studies, the estimations are qualitative, i.e. they
can only predict "good" or "bad" outcomes, but not
values of gait parameters. In addition, there is no re-
ported study about simulation of the effects of ham-
string lengthening on CP gait.
In this paper the effect of SEMLS on gait is evalu-
ated, in particular the effect of hamstring lengthening
on knee gait flexion at initial contact. Initial contact
is the stage of gait cycle when foot strikes the floor, as
338
Galarraga C. O., Vigneron V., Dorizzi B., Khouri N. and Desailly E..
Estimation of Postoperative Knee Flexion at Initial Contact of Cerebral Palsy Children using Neural Networks.
DOI: 10.5220/0005286503380342
In Proceedings of the International Conference on Pattern Recognition Applications and Methods (ICPRAM-2015), pages 338-342
ISBN: 978-989-758-077-2
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)
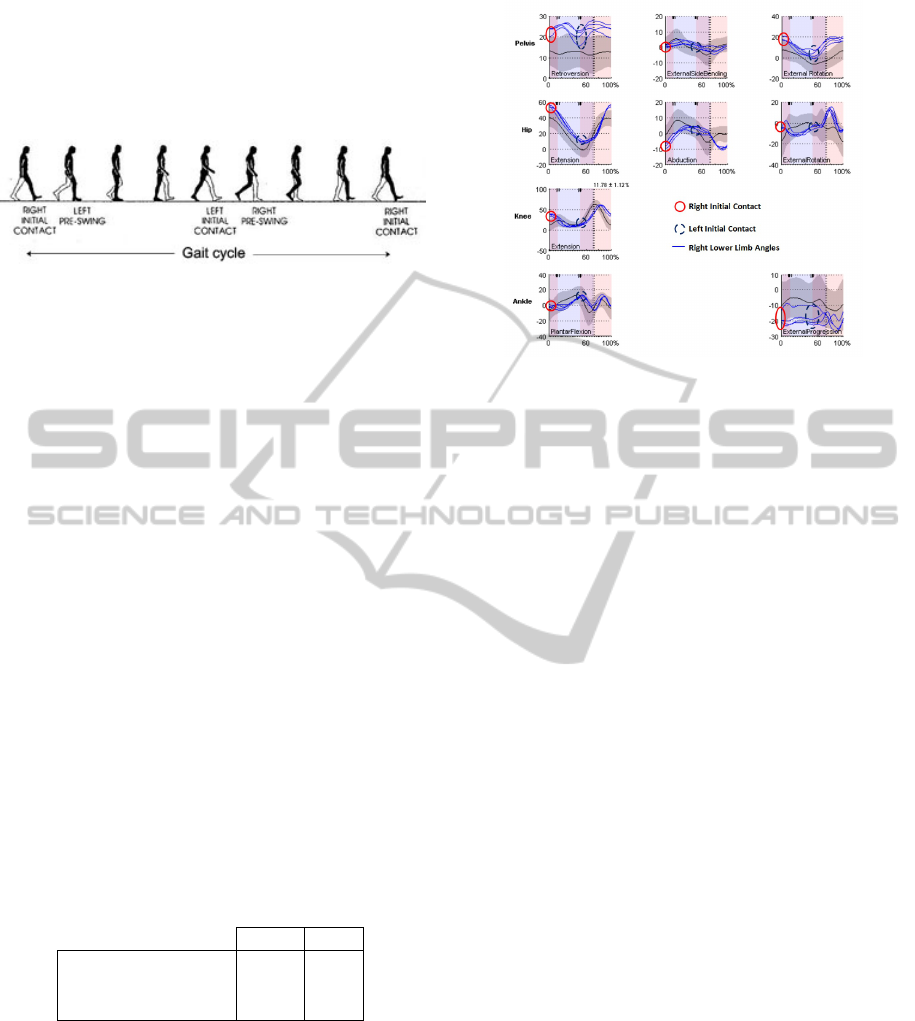
can be seen in figure 1.
The objective of this work is to design a simulator
that could be applied as a decision-making tool for
including or not the hamstring lengthening procedure
in a SEMLS context.
Figure 1: Gait cycle stages (gai, 2014 ).
To achieve the objective, supervised learning tech-
niques are applied on data of CP children that under-
went SEMLS (section 3). Post-surgical knee flexion
at initial contact is estimated knowing pre-surgical
gait and physical examination, given a surgery. The
nonlinear regression is performed by multi-layer
feedforward neural networks.
2 DATA DESCRIPTION
All the patients in the database have undergone
surgery and have had at least a CGA before and af-
ter the surgery. To simplify the problem, both sides
lower limbs are considered independently. In total
there are N = 193 limbs corresponding to N
pat
= 100
patients (7 limbs are not valid due to lack of informa-
tion whether on CGA or the physical exam). Male
subjects represent 61% of the population and 39% are
female. The mean ages and standard deviation of the
patients at the three different stages considered (Pre-
operative CGA, surgery and Postoperative CGA) can
be seen in table 1.
Table 1: Distribution of patients ages at CGAs and surgery.
µ σ
Preoperative CGA 11.80 3.30
Surgery 12.60 3.24
Postoperative CGA 14.76 3.32
For each CGA, we consider fifteen gait angles at
initial contact, as shown in figure 2. The mean of
each of these angles is computed when several gait
cycles are available (multiple initial contacts). Phys-
ical examinations provide preoperative popliteal an-
gles (PoplA).
We collect n = 714 walk cycles (n 193 because
we record several walk cycles per patient) and denote
our observation set by (x
1
, y
1
), . . . , (x
n
, y
n
) that may
be regarded as a finite realization of a multivariate
Figure 2: Considered gait angles. Example for the right
lower limb signals. The left lower limbs signals are also
considered at both sides initial contacts.
stochastic data collection. The parameters used for
the computation are defined as:
PTIC Pelvis Tilt
POIC Pelvis Obliquity
PRIC Pelvis Rotation
HFIC Hip Flexion
HAIC Hip Abduction
HRIC Hip Rotation
KFIC Knee Flexion
AFIC Ankle DorsiFlexion
FFIC Foot-Ground Flexion
HF
cont
IC Contralateral Hip Flexion
HA
cont
IC Contralateral Hip Abduction
HR
cont
IC Contralateral Hip Rotation
KF
cont
IC Contralateral Knee Flexion
AF
cont
IC Contralateral Ankle Flexion
FF
cont
IC Contralateral Foot Flexion
The above angles are in degrees and are measured at
initial contact (see figure 1).
3 METHOD
First, input variables are selected from all available
data. Second, a nonlinear regression of the post-
surgical knee flexion angle during gait is done by a
feedforward neural network. Leave-one-out cross-
validation (Bishop, 2006) is performed in order to
have a measure of the regression error for each patient
in the database.
The variable selection is done by a ranking tech-
nique adding a prone variable (Guyon and Elisseeff,
2003). The variables ranked higher than the prone are
selected as inputs for the neural network. Variables
ranked lower than the prone are rejected as entries.
EstimationofPostoperativeKneeFlexionatInitialContactofCerebralPalsyChildrenusingNeuralNetworks
339
The prone is a completely random variable unrelated
to the target output.
The ranking technique consists on a Gram-
Schmidt orthogonalization (Dreyfus et al., 2008;
Guyon and Elisseeff, 2003). If P
1
, . . . , P
k
are the k
vectors corresponding to the candidate variables, R is
the prone and Y is the target output vector, this it-
erative procedure is described by algorithm 1, where
hhA, Bii is the inner product of vectors A and B.
Algorithm 1: Gram-Schmidt variable ranking with prone.
1: P
k+1
← R
2: for i = 1 to k + 1 do
3: Z
i
← argmax
P
j
, j=1,...,k+1
Cov(P
j
,Y )
σ
P
j
σ
Y
4: for j = 1 to k + 1 do
5: P
j
← P
j
−
hhP
j
,Z
i
ii
hhZ
i
,Z
i
ii
Z
i
6: end for
7: Y ← Y −
hhY,Z
i
ii
hhZ
i
,Z
i
ii
Z
i
8: end for
At the first iteration in algorithm 1, the candidate
variable that is most correlated with the target output
is selected. Then all the candidate variables and the
target output are projected into the orthogonal space
of the selected variable. In this new space, again the
variable which is the most correlated to the target is
chosen. This process is repeated until all variables are
ranked.
The candidate variables are the fifteen preopera-
tive mean gait angles at foot strike and the preopera-
tive popliteal angle.
The selected variables are the input of the neural
network plus a binary input corresponding to the in-
clusion of hamstrings lengthening surgery. HL = 1
means that the hamstrings lengthening was performed
and HL = 0 means that another kind of surgery was
applied. The target output is the postoperative knee
flexion angle at initial contact (KFIC
post
).
The neural network architecture consists of a
multi-layer perceptron with one hidden layer. The
number of hidden units is optimized using the val-
idation error rates. The learning method is the
Levenberg-Marquardt algorithm (Bishop, 2006). Pre-
processing consists on centering and normalizing
data.
In order to have a measure of the error for all the
patients in the database, a neural network is trained
for each subject, then tested only for the subject in
question. For each neural network, the training set is
composed of all the available gait cycles, except for
those belonging to the patient that will be tested. Test
is performed only over the mean cycle angles per pa-
tient. The architecture of the neural networks is al-
ways identical.
The leave-one-out cross-validation (Duda et al.,
2001) is performed M = 10 and then the mean errors
per patients are calculated, as shown in algorithm 2.
The error measure considered for each patient i is the
root mean-square error (RMSE) computed as in equa-
tion 1.
Algorithm 2: Leave-one-out cross-validation.
1: for i = 1 to M do
2: for j = 1 to N
pat
do
3: Initialize NN
4: Train NN without patient j
5: Test patient j
6: end for
7: end for
8: Compute mean errors
RMSE
i
=
q
(Y
i
− g(X
i
))
2
= |Y
i
− g(X
i
)| (1)
4 RESULTS
From the list of variables in section 2, the selected
inputs ordered by relevance are: KFIC, FF
cont
IC,
PoplA, HR
cont
IC, AFIC, HA
cont
IC and PTIC.
Best results were obtained for m = 10 hidden
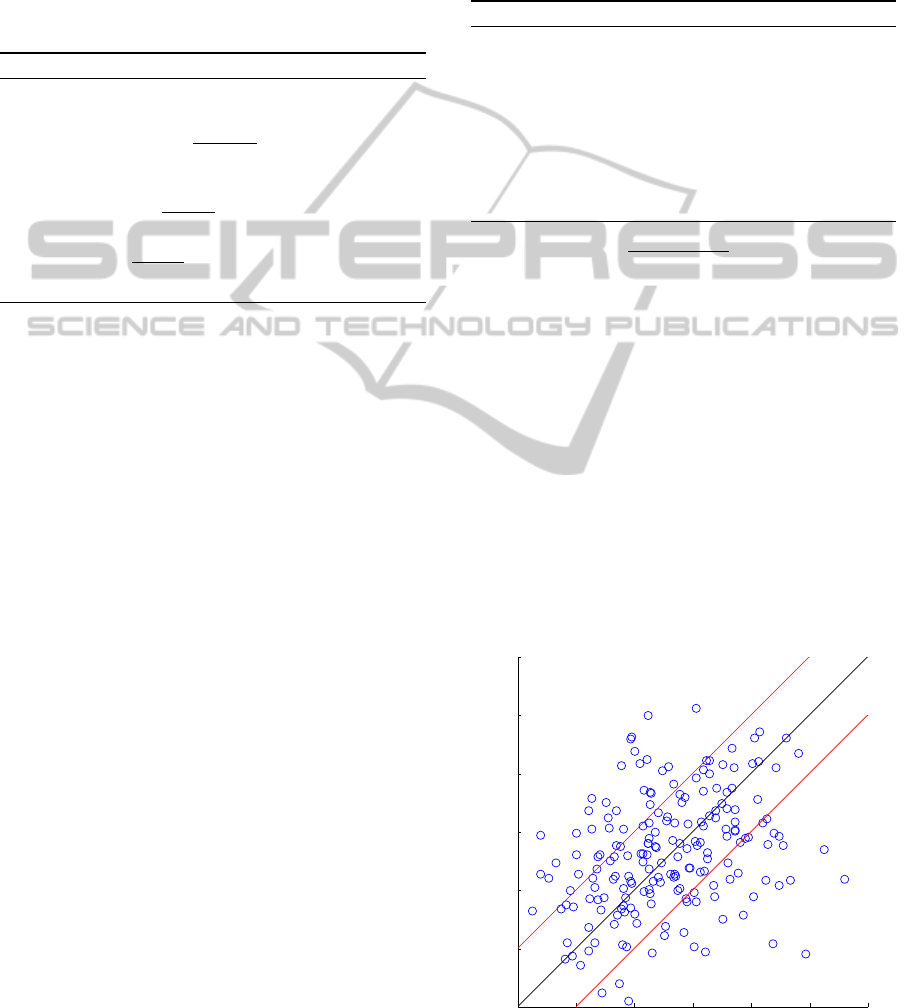
units. Figure 3 shows the estimated KFIC with re-
spect to the real KFIC
post
for one of the multiple
leave-one-out procedures (see section 3). The black
line corresponds to the ideal estimation and points
between the two red lines stay within a 10
◦
RMSE
range.
0 10 20 30 40 50 60
0
10
20
30
40
50
60
KFICpost
Estimated KFIC
Figure 3: Estimated vs. real post-surgical knee flexion.
ICPRAM2015-InternationalConferenceonPatternRecognitionApplicationsandMethods
340
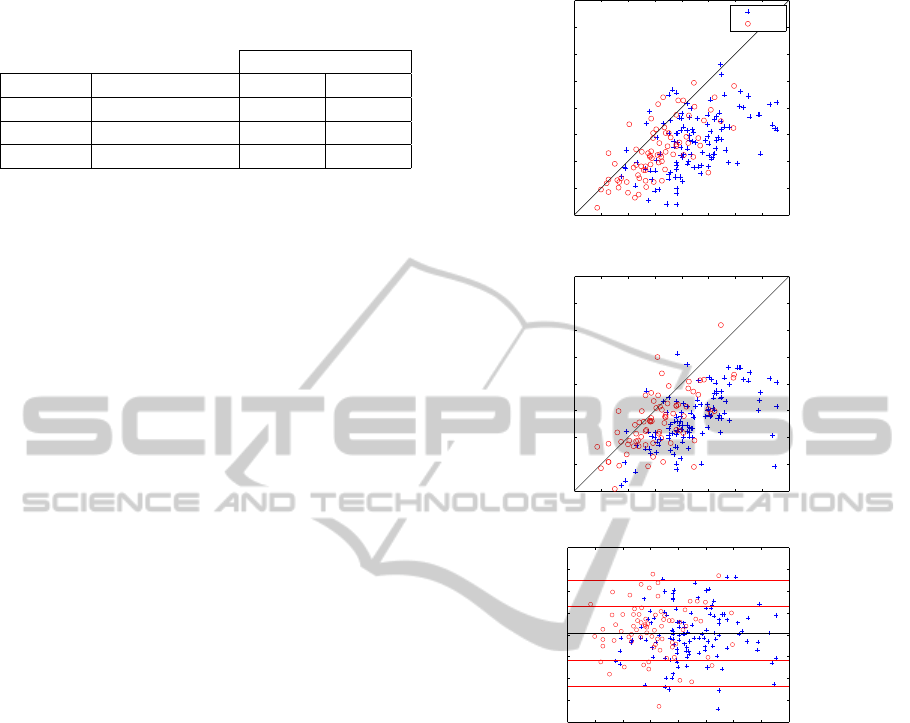
Table 2: Test results per patient and given the type of
surgery.
RMSE
Subjects µ
RMSE
± σ
RMSE
≤ 10
◦
≤ 15
◦
All 9.25
◦
± 5.45
◦
63.21% 86.53%
HL = 1 9.36
◦
± 5.68
◦
62.61% 85.22%
HL = 0 9.09
◦
± 5.13
◦
64.10% 88.46%
In table 2, mean RMSE and the corresponding
standard deviations can be observed for all patients,
those who underwent hamstring lengthening (HL =
1) and those who had another type of surgery. In
addition, percentages of subjects within 10
◦
and 15
◦
RMSE range are also given.
To show the relation between the outputs (target,
estimated and errors), figure 4 shows these three out-
puts with respect to the preoperative knee flexion at
initial contact. In 4(a) and 4(b), points over the black
line correspond to patients where the KFIC increased.
On the other hand, in figure 4(c), the black line rep-
resents the mean of the difference between estimated
KFIC and KFIC
post
. The red lines correspond to plus
and minus one and two standard deviation respec-
tively. In all the images previous mentioned, blue
crosses represent subjects that underwent hamstring
lengthening and red circles correspond to subjects that
had another kind of surgery.
5 DISCUSSION
The variable selection applied allows to decrease the
dimension of the problem from 16 to 7, which is
less than the half. This also decreases complexity of
the nonlinear regression. Moreover, with the Gram-
Schmidt orthogonolization, we maximize the corre-
lation of the input with the target output and, at the
same time, we reduce redundancy between the entry
variables.
Since surgical treatment implies various risks and
potential complications, a threshold for the difference
between estimated and measured postoperative knee
flexion has to be defined in order to determine wether
or not an estimation is acceptable. If we consider that
intrasubject gait variability is higher in CP children
than in normal children (Steinwender et al., 2000),
that the interlaboratory gait variabilty for knee flexion
is 13
◦
in (Noonan et al., 2003) and that there are un-
certainties associated to CGA (Charlton et al., 2004;
Groen et al., 2012) and surgery, we define as accept-
able an estimation with maximum 10
◦
of error. The
system estimates 63.21% of patients within this er-
ror range (see table 2). The error rates are almost the
same for both groups of patients: those who under-
0 10 20 30 40 50 60 70 80
0
10
20
30
40
50
60
70
80
KFICpost
HL=1
HL=0
(a)
0 10 20 30 40 50 60 70 80
0
10
20
30
40
50
60
70
80
Estimated KFIC
(b)
0 10 20 30 40 50 60 70 80
−40
−30
−20
−10
0
10
20
30
40
Estimated KFIC − KFICpost
KFICpre
(c)
Figure 4: Target, estimation and error with respect KFIC
pre
.
(a) KFIC
post
vs. KFIC
pre
. (b) Estimated KFIC vs. KFIC
pre
.
(c) Difference between estimated KFIC and KFIC
post
vs.
KFIC
pre
.
went hamstring lengthening and those who had an-
other surgery.
On the other hand, the distribution of points in fig-
ures 4(a) and 4(c) are similar, and they have the same
proportion of data above and below the black line.
This means that the system can forecast bad (above
the black line) and good outcomes. In figure 4(c), we
can observe that the systems overestimates and un-
derestimates uniformly with respect to preoperative
knee flexion at initial contact. Additionally, errors
are equally distributed in function of the KFIC
pre
in-
put independently if the subjects had or not hamstring
lengthening.
EstimationofPostoperativeKneeFlexionatInitialContactofCerebralPalsyChildrenusingNeuralNetworks
341

6 CONCLUSIONS
The proposed simulator can estimate the postopera-
tive knee flexion at initial contact, given the preopera-
tive gait, physical examination and a surgery. On test,
63.21% of the N = 193 limbs are estimated with an
acceptable regression error (see section 5). In addi-
tion, the mean RMSE is 9.25
◦
, which means that the
expected error of regression is also acceptable (infe-
rior to 10
◦
).
The developed system is able to give good estima-
tions independently of the preoperative gait parame-
ters and the type of surgery. However, around a third
of the patients are not well estimated. For the applica-
tion, it is important to apply surgery only if a good re-
sult can be asserted. Conversely, it is crucial to avoid
a surgery plan if a bad outcome is most likely. For
this reason, it would be interesting to detect a priori
patients that will be badly estimated. With this strat-
egy, patients more likely to be badly estimated, could
be rejected by the simulator. For example, if a new
patient is too far from the training patients in the in-
put space, no estimation will be given.
In order to improve estimation, a prior cluster-
ing of the patients could be applied, so the regression
method would be able to adapt to the type of subject.
Finally, this study predicts the outcomes of or-
thopaedic surgery with real gait parameters, and not
with qualitative parameters that are too relative and
ambiguous for such a sensitive application.
Further work will focus on estimating the whole
postoperative knee flexion gait cycle signal and not
only the initial contact point.
ACKNOWLEDGEMENTS
This work is part of a project funded by the Fondation
Ellen Poidatz, the Fondation Bettencourt Schueller
and the Region Ile de France. The authors would like
to thank the UNAM medical and technical team of the
Fondation Ellen Poidatz, who recorded all data used
in this work.
REFERENCES
Walking in graphs. http://www.utdallas.edu. Accessed:
2014-10-09.
Armand, S., Watelain, E., Roux, E., Mercier, M., and Lep-
outre, F.-X. (2007). Linking clinical measurements
and kinematic gait patterns of toe-walking using fuzzy
decision trees. Gait & Posture, 25(3):475–484.
Arnold, A. S., Liu, M. Q., Schwartz, M. H., Õunpuu, S.,
and Delp, S. L. (2006). The role of estimating muscle-
tendon lengths and velocities of the hamstrings in the
evaluation and treatment of crouch gait. Gait & Pos-
ture, 23(3):273–281.
Bishop, C. (2006). Pattern Recognition and Ma-
chine Learning. Information Science and Statistics.
Springer.
Charlton, I., Tate, P., Smyth, P., and Roren, L. (2004). Re-
peatability of an optimised lower body model. Gait &
Posture, 20(2):213–221.
Desailly, E. (2008). Analyse biomécanique 3D de la marche
de l’enfant déficient moteur. Modélisation segmentaire
et modélisation musculo-squelettique. PhD thesis,
Université de Poitiers.
Dreyfus, G., Martinez, J.-M., Samuelides, M., Gordon, M.,
Badran, F., and Thiria, S. (2008). Apprentissage statis-
tique. Eyrolles, Paris, 3rd edition.
Duda, R., Hart, P., and Stork, D. (2001). Pattern Classifica-
tion. Wiley-Interscience, 2nd edition.
Gage, J., Schwartz, M., Koop, S., and Novacheck, T., edi-
tors (2009). The Identification and Treatment of Gait
Problems in Cerebral Palsy. Mac Keith Press, 2nd
edition.
Groen, B., Geurts, M., Nienhuis, B., and Duysens, J.
(2012). Sensitivity of the OLGA and VCM models to
erroneous marker placement: Effects on 3d-gait kine-
matics. Gait & Posture, 35(3):517–521.
Guyon, I. and Elisseeff, A. (2003). An introduction to vari-
able and feature selection. The Journal of Machine
Learning Research, 3:1157–1182.
Ma, F. Y. P., Selber, P., Nattrass, G. R., Harvey, A. R.,
Wolfe, R., and Graham, H. K. (2006). Length-
ening and transfer of hamstrings for a flexion de-
formity of the knee in children with bilateral cere-
bral palsy TECHNIQUE AND PRELIMINARY RE-
SULTS. Journal of Bone & Joint Surgery, British Vol-
ume, 88(2):248–254.
Noonan, K. J., Halliday, S., Browne, R., O’Brien, S., Kayes,
K., and Feinberg, J. (2003). Interobserver variability
of gait analysis in patients with cerebral palsy. Journal
of Pediatric Orthopaedics, 23(3):279–287.
Reinbolt, J. A., Fox, M. D., Schwartz, M. H., and Delp,
S. L. (2009). Predicting outcomes of rectus femoris
transfer surgery. Gait & Posture, 30(1):100–105.
Sebsadji, A., Khouri, N., Djemal, K., Yepremian, D., Hareb,
F., Hoppenot, P., and Desailly, E. (2012). Description
and classification of the effect of hamstrings lengthen-
ing in cerebral palsy children multi-site surgery. Com-
puter Methods in Biomechanics and Biomedical Engi-
neering, 15(sup1):177–179.
Steinwender, G., Saraph, V., Scheiber, S., Zwick, E. B.,
Uitz, C., and Hackl, K. (2000). Intrasubject repeata-
bility of gait analysis data in normal and spastic chil-
dren. Clinical Biomechanics, 15(2):134–139.
ICPRAM2015-InternationalConferenceonPatternRecognitionApplicationsandMethods
342
