
Development of an Automated System for Ex Vivo Measuring the
Neuro Muscular Junction Functionality
Simona Pisu
1
, Emanuele Rizzuto
2
, Antonio Musarò
1
and Zaccaria Del Prete
2
1
DAHFMO-Unit of Histology and Medical Embryology, Sapienza University of Rome, Rome, Italy
2
Department of Mechanical and Aerospace Engineering, Sapienza University of Rome, Rome, Italy
1 RESEARCH PROBLEM
The present work is part of a research project, which
aims to characterize the nerve-muscle interaction in
Amyotrophic Lateral Sclerosis (ALS), a fatal
neuromuscular disease associated with motor neuron
degeneration, muscle atrophy and paralysis. The loss
of connection between muscle and nerve is a crucial
biological mechanism severely impaired in ALS. In
this context, to investigate the alterations in the
communication between muscle fibers and motor
neurons, we started studying the neuro muscular
junction (NMJ) from a functional point of view. The
capability of measuring the NMJ functionality can
therefore give essential information on its physio-
pathological conditions. Therefore, this study may
be useful to discriminate damages of the different
motor unit components in neuromuscular diseases
and, in long-term, to design more appropriate
therapeutic approaches.
In particular, the result that we expect to achieve
is to better clarify which element of the motor unit is
initially affected by this disease. In fact, the recently
proposed “dying-back” hypothesis supports the idea
that the damages begin in the NMJ and then spread
towards the motor neuron body, in opposition to the
more traditional idea according to which the muscle
is only a secondary target of the disease.
2 OUTLINE OF OBJECTIVES
In the described context, the aim of my PhD project
is to characterize the functionality of the
communication between muscle and nerve in
pathological mouse models, by developing new
automated experimental methodologies.
The measurement of NMJ functionality is
obtained by comparing muscle contractile response
elicited by nerve stimulation (indirect), with the
response of the same specimen to membrane
stimulation (direct). Since this latter stimulation
bypasses the neuronal signalling, any difference
between the two contractile responses may be
related to NMJ alterations. To date, I started
working with Soleus muscle-nerve specimens of
healthy Wild Type mice, to develop an experimental
system for studying NMJ functionality of ex vivo
muscle-nerve preparations. After that I’m
approaching the study of a ALS mouse model. In the
future years, I will approach the realization of new
experimental systems for testing NMJ functionality
in isotonic conditions and investigate directly in vivo
the muscle behavior. In all the systems We aim to
develop we will pay special attention to the accuracy
and the repeatability of the experimental procedures.
These metrological qualities will counteract the
negative effects due to the high variability that
usually arises when working with biological tissues.
3 STATE OF THE ART
To better define the alterations in the coupling
between motor neuron conduction and muscle
contraction we started evaluating the physio-
pathologic properties of both skeletal muscle and
NMJ, by stimulating the muscle directly and
indirectly. In particular, we studied standard muscle
contractile properties, as previously described by us
and by other research groups (Brooks SV
and
Faulkner JA, 1988; Del Prete et al, 2008). On the
other hand, although the NMJ functionality
technique has been extensively used on rats (Aldrich
et al., 1986; Van Lunteren and Moyer, 2004), only a
few works have been attempted on pathological
mouse models: Personius et al. measured the
diaphragm NMJ functionality of adult dystrophic
mdx mice (Personius and Sawyer, 2006), while Lee
et al. (Lee et al., 2011) and Ling et al. (Ling et al.,
2009) measured the NMJ properties respectively in
36
Pisu S., Rizzuto E., Musarò A. and Del Prete Z..
Development of an Automated System for Ex Vivo Measuring the Neuro Muscular Junction Functionality.
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)
Soleus and EDL of an animal model of spinal
muscle atrophy at a few days after birth. This
literature has been taken as a starting point, but these
studies are based on animal models of different ages
and sizes or different muscles, if compared with
ours. For this reason it was not possible to employ
the stimulation parameters as proposed.
The pathological model we decided to study at
first is the SOD1
G93A
mice (Gurney et al., 1994),
one of the most studied animal model of ALS
(Turner and Talbot, 2008). However, its skeletal
muscles contractile properties have been poorly
investigated and the NMJ functionality at all.
Previous studies reported a deficit in the generation
of maximum force in hind limbs muscles of
SOD1
G93A
mice, when compared to wild type
littermates (Hegedus et al., 2008; Derave et al.,
2003). Some authors detected a reduced number of
motor neurons associated with disruption of the
neuromuscular junction (Ngo et al., 2012; Fischer et
al, 2003).
Temporal analysis of axon and NMJ
degeneration in transgenic mice indicate that
motorneuron pathology begins distally from the
synaptic area, and then proceeds towards soma in a
retrograde dying back manner
(Luc Dupuis and Jean-
Philippe Loeffler, 2009).
4 METHODOLOGY
To the proposed aims, I have developed a system to
measure, ex vivo, the muscle contractile response
due to indirect stimulation trough the nerve and the
muscle contractile response due to direct membrane
stimulation. The proposed system is fully automated,
through the use of a custom-made software, that
allows to precisely control the experiment, making
all the tests repeatable and accurate.
4.1 Experimental Setup
The muscle to be tested is excised with its intact
innervation and vertically mounted in an oxygenated
(95% O
2
and 5% CO
2
) and temperature controlled
chamber (30°C), containing a bicarbonate-buffered
solution at pH 7.4. One end of the muscle is linked
to a fixed clamp and the other end is connected to
the lever-arm of an Aurora Scientific Instruments
Inc. (ASI) 300B actuator/transducer. The apparatus
allows to stimulate the muscle both directly and
indirectly. For direct stimulation, two platinum
electrodes are located 2 mm from each side of the
muscle and the electrical stimuli are current pulses
of 300 mA generated by an ASI 701C stimulator.
For indirect stimulation, the nerve is sucked into a
suction electrode (A-M Systems Inc.) and
supramaximal pulses were delivered to the nerve by
an ASI 701B stimulator.
4.2 Protocol
We have developed a protocol that allows to study in
Soleus muscles several parameters of the isometric
muscular contraction and of the NMJ functionality,
proposed in the literature, in a single test. Indeed,
Personius et al. applied the fatigue protocols at two
frequencies on different specimens and van Lunteren
et al. studied the intratetanic fatigue, using separate
repetitive stimulations, delivered or directly on the
muscle or through the nerve.
The protocol is composed of four parts. Initially,
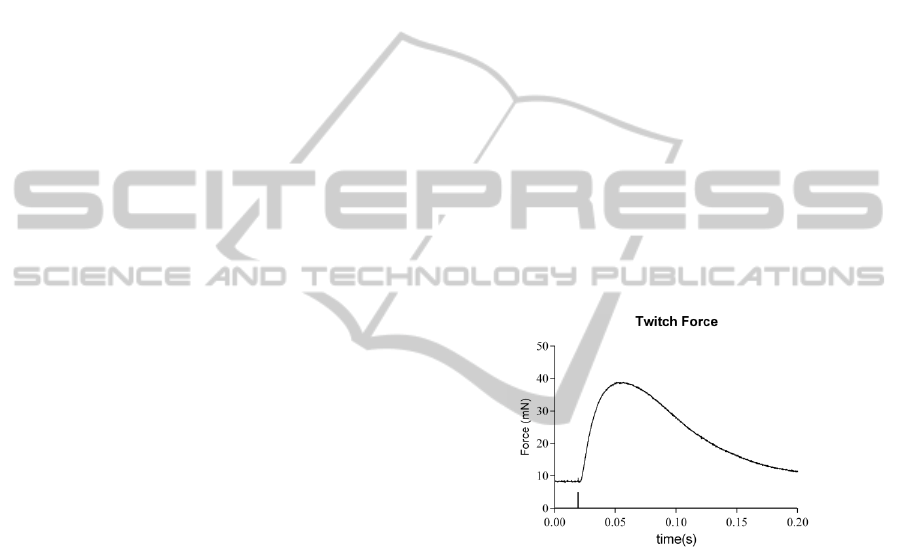
the muscle is stimulated with 4 single pulses to
measure twitch force (F
tw
) and kinetic parameters,
namely time to peak (TTP), half relaxation time
(1/2RT) and maximum value of force time
derivative (dF/dt), for both membrane and nerve
stimulations, as shown in figure 1.
Figure 1: Twitch force (Ftw), time to peak (TTP), half
relaxation time (1/2RT) and maximum value of force time
derivative (dF/dt) can be measured with single pulse
stimulation.
After that, 8 shuffled stimulations in the range
between 20 Hz, which is the lowest summation
frequency, and 80 Hz, the Soleus tetanic frequency,
are used to measure the force-frequency curves for
both muscle and nerve stimulations. Finally, the
NMJ functionality parameters, namely
Neurotransmission Failure (NF) and Intratetanic
Fatigue (IF), are measured with two isometric
fatigue paradigms. These paradigms are made up of
14 stimulations via the nerve and 1 stimulation on
the membrane, to induce a specific stress in the
NMJ. The first paradigm is based on pulse trains
delivered at a physiological firing frequency of
35 Hz while the second is composed of pulse trains
delivered at the tetanic frequency. We employed
DevelopmentofanAutomatedSystemforExVivoMeasuringtheNeuroMuscularJunctionFunctionality
37

0.8 s pulse trains, with a rest period of 1.2 s between
each of them, to stimulate the soleus, differently
from Personius et al. that used 0.33s pulse train to
stimulate the diaphragm membrane and the phrenic
nerve, with a rest period of 0.67s after each train.
This differences are due to the different fiber
composition of the two muscles. This sequence is
repeated 20 times, for a total paradigm of 10 min.
The Neurotransmission Failure parameter
compares the force decrease due to the nerve
conduction to that due to muscle contraction during
the paradigm.
where F is the force decrease after nerve stimulation
and MF is the force decrease after membrane
stimulation .
Intratetanic Fatigue represents the force drop
which can occur within the same tetanic contraction,
in case of stimulation via the nerve.
where F
LP
is the force at the last pulse and F
MAX
is
the maximum value of force within the single train
of pulses (see figure 2).
Figure 2: Intratetanic Fatigue is the force decrease within
the same pulse train. From van Lunteren et al., 2004.
4.3 Software
The experimental setup is controlled by a Windows
PC and a custom-made software in LabView 2011.
The interface between the computer and the
equipment is managed by the National Instruments
data acquisition board NI PCI Express 6353. This
choice allows to have a high flexibility in the
management of the experiments. The software is
used to control synchronously and automatically the
pulse stimulators and the actuator/transducer. In
particular, all the stimulation parameters are
manageable by means of simple text files provided
to the software as input.
During the experiment the software
automatically changes the acquisition frequency
setting it to 20 kHz in case of stimulation with single
pulses, and to 1 kHz during the remaining
stimulations. This choice is due to the fact that the
temporal parameters measured during a single pulse
stimulation are of the order of tens of milliseconds,
and an accuracy of 50s is therefore needed to point
out any significant difference. During stimulations at
frequencies higher than the single pulse, no temporal
parameters are calculated, therefore is sufficient to
acquire the data at 1 kHz to correctly sample the
signals of interest avoiding an overloading of the
system.
The software so designed and realized is
extremely flexible and functional. In fact, it is able
to perform all the main tests to define the
mechanical characteristics of muscle tissue and NMJ
functionality. This versatility has been extremely
useful when carrying out the preliminary tests aimed
at determining the optimal stimulation parameters,
such as the durations of the pulses, the stimulation
frequencies and the waiting times. On the other
hand, during the trial it is possible to perform
different protocols in a simple way.
5 EXPECTED OUTCOME
The proposed protocol allows the measurement of
the NMJ functionality in Soleus muscle of any
mouse model, by testing isometric contraction. We
expect to be able to characterize the NMJ
functionality of other muscle types by the
application of this protocol with only minor
modifications.
5.1 Future Plans
To perform a functional characterization of the
muscle-nerve preparations that better represents the
muscle and NMJ in vivo behavior, I am confident of
being able to develop a similar methodology to
study the muscle isotonic contraction. To do this, a
tool which allows the suction electrode for nerve
stimulation to follow the muscle shortening will be
designed and realized. I am also going to develop a
in vivo/in situ methodology that can allow to study
different muscles or muscular groups to give
information on the nerve conduction in addition to
NMJ and muscle functionality. Based on this latter
technique, the long-term goal is to study a possible
improvement of muscle functionality following
stimulation training protocols.
BIOSTEC2015-DoctoralConsortium
38
6 STAGE OF THE RESEARCH
6.1 Setting Stimulation Parameters
To determine the optimal pulse parameters to
stimulate the sciatic nerve I started performing
preliminary tests on Soleus muscle-nerve
preparations of four months-old Wild Type mice. At
first, I checked the current intensity necessary to
elicit the nerve stimulation: this was found to be
between 5 mA and 10 mA. The lower limit is the
first current intensity that causes the maximum
twitch contraction force. Increasing the current sent
through the suction electrode beyond 10 mA it
generates a current field so intense as to induce also
the direct stimulation of the muscle membrane
through the solution. Event that should absolutely be
avoided.
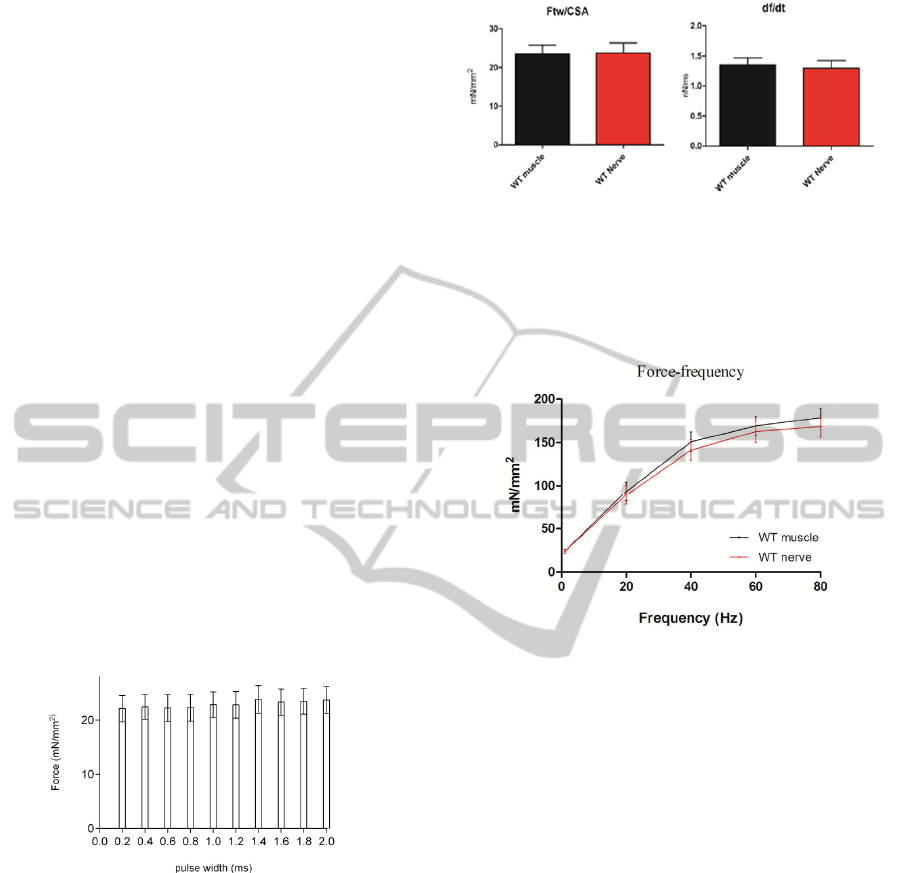
Once set the optimal current value, I looked for
the relation between the width of single pulses and
the twitch specific force. To this purpose 10 Wild
Type Soleus specimens were stimulated with single
pulses of widths between 0.2 ms and 2 ms, the range
most used in the literature. There was no significant
variation of specific force varying the pulses length.
However, in correspondence of 1.4 ms duration the
forces showed the highest value, as shown in figure
3.
Figure 3: Twitch specific forces developed stimulating via
the nerve with pulses of width between 0.2 and 2 ms.
The choice has been also supported by tests of
tetanic stimulation in which trains of pulses of 1.4
ms caused a developed force on average higher than
the other widths.
Once we have determined the two parameters of
the stimulation pulse, we moved to verify the
validity of the method. In a healthy specimen is
expected that direct and indirect stimulations bring
the muscle to contract in the same manner and
develop the same forces. In fact, as shown in figure
4, the single pulse tests showed the same twitch
force and kinetic contraction parameters in both
cases of stimulation: the direct and the indirect one.
Figure 4: Twitch force and df/dt of WT Soleus stimulated
directly and indirectly.
The force-frequency relations are also
comparable in both cases of stimulation (see figure
5).
Figure 5: Force-frequency curves from WT Soleus directly
and indirectly stimulated.
The fatigue paradigm is derived from Personius
et al., however the Soleus stimulation parameters
were modified accordingly to its fiber composition
and to the standard membrane stimulation
parameters. Since the Soleus muscle is composed by
a higher percentage of slow fibers than the
diaphragm, it needs a longer stimulation time to
develop the maximum force. This stimulation time is
estimated to be 0.8 s and 1 s is necessary to the
muscle relaxation. Therefore, the fatigue paradigms
were set as follows: one 0.8 s pulse train delivered
directly on the membrane, followed by fourteen
0.8 s pulse trains delivered through the nerve, with a
rest period of 1.2 s after each pulse train. By
repeating this series of stimulations for 20 times, as
for the diaphragm, each paradigm takes 10 minutes.
Some preliminary tests showed that a rest time of
15 min is necessary between the 35 Hz protocol and
the 80 Hz one to have repeatable results not affected
by muscle fatigue. The rest time that the specimens
needed to recover their physiological properties after
the first fatigue paradigm was considered adequate
when the force generated by the muscle at the
DevelopmentofanAutomatedSystemforExVivoMeasuringtheNeuroMuscularJunctionFunctionality
39
beginning of the second fatigue paradigm was at
least 90% of its maximum force.
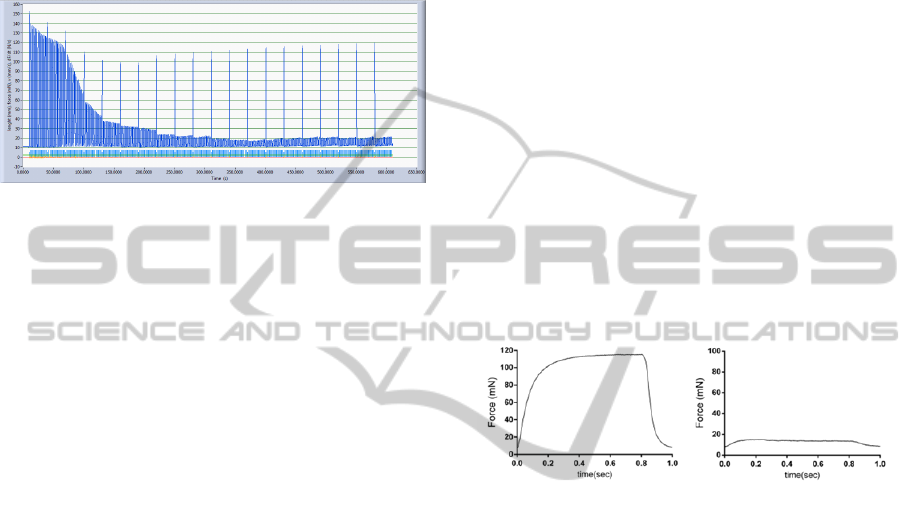
The application of the fatigue protocol showed
that the force developed by nerve stimulation
decreases very rapidly while the muscle continues to
be able to produce a much greater force as result of
direct stimulation, as shown in figure 6.
Figure 6: Example of a fatigue paradigm.
The calculation of the Neurotransmission Failure
gave results comparable to those existing in the
literature for the WT muscles. Likewise was not
revealed Intratetanic Fatigue in case of direct
stimulation of the muscle. On the other hand, in case
of indirect stimulation the IF is similar to that shown
by van Lunteren.
6.2 Transgenic Model
Once set the method on the wild type model, to
focus our attention on a pathologic model we started
studying the Soleus of SOD1
G93A
mice at the end
stage of the disease. At this age, in fact, significant
NMJ damages are expected thus allowing to obtain a
further confirmation of the validity of the method
and to test its sensitivity. The first experimental
results showed that transgenic muscles presents a
significant slowdown of the kinetic parameters if
directly stimulated, and a further slowdown in case
of nerve stimulation.
Analysis of force-frequency curves revealed
significant differences between WT and transgenic
muscles. At the tetanic frequency a decrease of
about 20% was reported for SOD1
G93A
Soleus
specific force, in comparison to the controls. Once
again, a worsening in the TG Soleus response was
reported when stimulated through the nerve. A
significant decrease of specific forces was, in fact,
measured for all the tested frequencies. The
maximum specific force developed in case of nerve
stimulation appears halved if compared to the direct
stimulation. These results highlight the sensitivity of
the method to discriminate even small temporal
differences and to separate the components of
muscle contraction due to muscle inner damages
from the ones due to NMJ conduction defects.
The analysis of the forces measured during the
80 Hz fatigue paradigm pointed out a limitation of
the method when calculating intratetanic fatigue at
the end of the protocol. In fact, in literature this
parameter is measured during repeated nerve
stimulations of about 2.5 minutes. We tried to
calculate the IF during the entire fatigue protocol
and we observed that at the beginning the generated
forces are always of the order of tens of mN so it is
possible to reliably measure the force decrease
within the same pulse train. On the contrary, at the
end of the same paradigm, the muscles were
exhausted and the contraction forces developed in
case of nerve stimulation were near to zero,
especially in the transgenic model. In this situation,
the sensitivity of the method resulted heavily
reduced, and the IF values calculated from the
seventh minute on, were the forces are about 7 mN,
basically expresses noise. For this reason we are
evaluating to shorten the paradigm. See figure 7.
Figure 7: Maximum force developed by the same
specimen at the first nerve stimulation end at the last nerve
stimulation during the fatigue paradigm.
Because of this reason, I decided to compute NF
and IF only in the first seven minutes of stimulation
of the 80 Hz fatigue protocol. Results showed higher
IF values, starting from the first stimulations, in
transgenic muscles stimulated trough the nerve
compared to the WT model.
On the contrary the analysis of
Neurotransmission Failure did not show any
alteration in the transgenic model. A possible
explanation of this may be that at the end-stage of
the pathology the transgenic
skeletal muscles are
also severely compromised and can hide the NMJ
defects.
In conclusion, the proposed experimental
technique allows to determine the NMJ functionality
separately from the muscle contractile properties in
isolated muscle-nerve preparations of pathological
mouse models. Preliminary results obtained from the
SOD1
G93A
model are in accordance with the
literature, showing muscle contraction defects and
NMJ impairment.
BIOSTEC2015-DoctoralConsortium
40

REFERENCES
Aldrich, T. K., Shander, A., Chaudhry, I., and Nagashima,
H. (1986). Fatigue of isolated rat diaphragm: role of
impaired neuromuscular transmission. Journal of Ap-
plied Physiology, 61(3):1077–1083.
Brooks, S. V. and Faulkner, J. A. (1988). Contractile prop-
erties of skeletal muscles from young, adult and aged
mice. Journal of Physiology, 404:71–82.
Del Prete, Z., Musaro`, A., and Rizzuto, E. (2008).
Measur- ing mechanical properties, including isotonic
fatigue, of fast and slow MLC/mIgf-1 transgenic
skeletal mus- cle. Annals of biomedical engineering.
Derave, W., Van Den Bosch, L., Lemmens, G., Eijnde, B.
O., Robberecht, W., and Hespel, P. (2003). Skele- tal
muscle properties in a transgenic mouse model for
amyotrophic lateral sclerosis: effects of creatine treat-
ment. Neurobiology of disease, 13(3):264–272.
Dupuis, L., Gonzalez de Aguilar, J. L., Echaniz-Laguna,
A., Eschbach, J., Rene, F., Oudart, H., Halter, B.,
Huze, C., Schaeffer, L., Bouillaud, F., and Loeffler, J.
P. (2009). Muscle mitochondrial uncoupling
dismantles neuromuscular junction and triggers distal
degenera- tion of motor neurons. PloS one,
4(4):e5390.
Fischer, L. R., Culver, D. G., Tennant, P., Davis, A. A.,
Wang, M., Castellano-Sanchez, A., Khan, J., Polak,
M. A., and Glass, J. D. (2003). Amyotrophic lateral
sclerosis is a distal axonopathy: evidence in mice and
man. Experimental Neurology, 185(2):232–240.
Gurney, M. E., Pu, H., Chiu, A. Y., Canto, M. C. D., Pol-
chow, C. Y., Alexander, D. D., Caliendo, J., Hentati,
A., Kwon, Y. W., and Deng, H. X. (1994). Mo- tor
neuron degeneration in mice that express a hu- man
Cu,Zn superoxide dismutase mutation. Science,
264(5166):1772–1775.
Hegedus, J., Putman, C. T., Tyreman, N., and Gordon, T.
(2008). Preferential motor unit loss in the SOD1 G93A
transgenic mouse model of amyotrophic lateral
sclerosis. The Journal of physiology, 586(14):3337–
3351.
Lee, Y. I., Mikesh, M., Smith, I., Rimer, M., and
Thompson, W. (2011). Muscles in a mouse model of
spinal mus- cular atrophy show profound defects in
neuromuscu- lar development even in the absence of
failure in neu- romuscular transmission or loss of
motor neurons. De- velopmental biology, 356(2):432–
444.
Ling, K. K. Y., Lin, M.-Y., Zingg, B., Feng, Z., and Ko,
C.-P. (2009). Synaptic defects in the spinal and neu-
romuscular circuitry in a mouse model of spinal mus-
cular atrophy. PloS one, 5(11):e15457–e15457.
Ngo, S. T., Baumann, F., Ridall, P. G., Pettitt, A. N.,
Henderson, R. D., Bellingham, M. C., and Mc-
Combe, P. A. (2012). The relationship between
Bayesian motor unit number estimation and histo-
logical measurements of motor neurons in wild-type
and SOD1(G93A) mice. Clinical Neurophysiology,
123(10):2080–2091.
Personius, K. E. and Sawyer, R. P. (2006). Variability and
failure of neurotransmission in the diaphragm of mdx
mice. Neuromuscular disorders : NMD, 16(3):168–
177.
Turner, B. J. and Talbot, K. (2008). Transgenics, toxicity
and therapeutics in rodent models of mutant SOD1-
mediated familial ALS. Progress in neurobiology,
85(1):94–134.
Van Lunteren, E., Moyer, M., and Kaminski, H. J. (2004).
Adverse effects of myasthenia gravis on rat phrenic di-
aphragm contractile performance. Journal of Applied
Physiology, 97(3):895–901.
DevelopmentofanAutomatedSystemforExVivoMeasuringtheNeuroMuscularJunctionFunctionality
41
