
Enhanced Stability and Re-usability of the Optical Sensor for pH
Monitoring Using a Layer-by-layer Deposition Technique
Nahid Raoufi
1,2
, Frederic Surre
2
, Muttukrishnan Rajarajan
2
, Tong Sun
2
and Kenneth T. V. Grattan
2,3
1
Engineering Department, Islamic Azad University – South Tehran Branch, Tehran, Iran
2
School of Engineering and Mathematical Sciences, City University London, London, EC1V 0HB, U.K.
3
City Graduate School, City University London, London, EC1V 0HB, U.K.
Keywords: Stabilization, Stable, Layer-by-layer, Sensor, pH Optical Sensor, Re-usability.
Abstract: Stable and reliable pH optical sensor is important for many industrial applications. The layer-by-layer
deposition technique is a simple and versatile method used to deposit a sensitive thin film on such an optical
fibre-based device but creating a coating which can often be destroyed in use in highly acid or alkali solutions.
It is thus important to create stable and durable sensors for operation under these extreme environments. The
main aim of this study has been to prepare a number of such sensors and compare the performance of three
different stabilization approaches used for the development of an effective wavelength-dependent pH-
sensitive optical sensor. Techniques such as employing heat treatment, the deposition of two layers of a
PAH/SiO
2
thin film and the deposition of two layers APTMS/SiO
2
as topping layers have been studied to
determine the optimum approach to creating a desirable sensor – one yielding the same value of peak
wavelength for a measurement of a known value of pH and to do so repeatedly. An improvement in
performance and in shelf-life, stability and re-usability of the sensor has been achieved by the addition of two
bilayers of APTMS/SiO
2
in the work carried out and the results of the investigation undertaken are reported.
1 INTRODUCTION
Nowadays, the detection of a range of chemical and
biochemical substances, as well as the measurement
of a variety of system operating parameters play an
important role in many situations. pH is one of the
most common analytical measurements needed in
both industrial processing and in laboratory research,
in which reliable real time sensor data, such as can be
obtained from an optical sensor system due to its light
weight and non-electrical mode of operation is
needed. In order to achieve optical recognition of
these parameters using optical fibre-based devices,
active indicators such as sensitive films must be
immobilized on the distal ends of suitable optical
fibres. The layer-by-layer technique is one of the
deposition methods widely used to coat such thin
films on to optical substrates and optical fibres. The
layer-by-layer (LbL) technique is used to build up a
sufficient thickness of such material on the fibre and
is based on the electrostatic attraction between
oppositely charged molecules to create the layers and
thereby increase the overall coating thickness
(Decher et al., 1992). The principal advantage of the
use of this technique is the ability to create stable
deposited thin films with well-organized structure
and controlled nanometer thicknesses on substrates of
various shapes and sizes (Decher and Schlenoff,
2002, Ai et al., 2003, Johnston et al., 2006,
Cassagneau et al., 1998, Dubas et al., 2006).
Generally, the thin films created by using the LbL
technique are stable (de Villiers et al., 2011, Decher
and Schlenoff, 2002), and it is difficult to remove
them from a solid substrate. There are two main
methods to remove LbL deposited films, should this
be needed. First, a solution of high pH can be used
which will attack the first polycation layer and
destroy the ionic bonds that stabilize the films. A
second method is to expose the LbL multilayers to a
solution with very high ionic strength. For practical
applications, especially those needing continuous
monitoring, it is critical to have a pH probe that can
give consistent results and survive for as long as
possible. However, the destruction of the layers limits
the life of the probe and does not make it as suitable
for continuous monitoring. A variety of techniques
has been proposed to improve the stability of the film
and to avoid progressive destruction of the coating.
Ionic strength, pH, concentration of the polyion
solutions and the presence of a copolymer such as salt
156
Raoufi N., Surre F., Rajarajan M., Sun T. and Grattan K..
Enhanced Stability and Re-usability of the Optical Sensor for pH Monitoring Using a Layer-by-layer Deposition Technique.
DOI: 10.5220/0005432401560167
In Proceedings of the 3rd International Conference on Photonics, Optics and Laser Technology (OSENS-2015), pages 156-167
ISBN: 978-989-758-092-5
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)

affect the LbL assembly, the film thickness and its
stability (Ai et al., 2003, Decher and Schlenoff,
2002). Heat treatment is also an important process
which has been discussed in many works in the
literature (Sharma et al., 2001, Singh et al., 2008,
Decher et al., 1994) to achieve a higher stability of the
thin films and avoid problems with the destruction of
the films when they are immersed in buffer solutions
of different values of pH (Villar et al., 2005, Villar et
al., 2008). However this sort of treatment affects the
sensor performance and decreases its sensitivity
(Villar et al., 2008), as well as allowing for a
degradation of the indicator dyes used which happens
at high temperatures.
The stability of thin films thus generated does
depend on the interaction between the layers, such as
through the formation and destruction of hydrogen
bonds. Hence, the stabilization of the LbL-assembled
films via polyamide bond formation was a further
method reported in the literature (Ichinose et al.,
1999, Yamada et al., 1981, Li et al., 2005). The amine
coupling reaction can easily allow a cross-linkage of
an amino group to a cationic polyelectrolytes and a
carboxyl group on anionic polyelectrolytes via amide
bond formation. There is a further report in the
literature (Saeki et al., 2013) which focused on the
film stability under chlorine treatment as a means to
improve the stability of the LbL-assembled
nanofilteration membranes in combined high ionic
strength conditions and under chlorine treatment. In
another approach, the stabilizing of the thin film is
achieved by forming siloxane bonds owing to a silane
coupling reaction between oppositely charged
polyelectrolytes which leads to the crosslinking
between the silane groups (Sen et al., 1992, Saeki et
al., 2013). Egawa et al. demonstrated (Egawa et al.,
2007) crosslinking between the sulfonate group in
the polyanion and the diazonium ion in polycation
due to exposure to UV light. The pH sensor reported
in their work could be used to measure solutions of
high pH.
An alternative approach is to build up several capping
layers using different materials such as nanoparticles
to enhance the film stability. Prakash et al. (Prakash
et al., 2013) discussed applying nanoparticles to
achieve an adequate sensitivity and stability with the
modification of the sensors (or biosensors) with
nanomaterials such as gold and/or silver
nanoparticles (Su and Li, 2008, Dubas et al., 2006),
carbon nanomaterials (Llobet, 2013) and silica
nanoparticles (Wang et al., 2013) and these have
shown considerable promise. Putzbach et al. reported
that the immobilization of enzymes improves stability
of the biosensor discussed (Putzbach and Ronkainen,
2013).
The application of silica nanoparticles (SiO
2
) has
drawn considerable attention for surface
modification, due to its uses as an enhancer for the
sensitivity, selectivity and strength of the thin films as
well as a its use as a pH indicator in many research
situations and in industry (Liu and Cui, 2007,
Nallathambi et al., 2011, Potapov et al., 2011, Lee and
Cui, 2012, Rahman and Padavettan, 2012). The work
presented in this paper thus takes advantage of this
and compares and contrasts three different
stabilization approaches with the aim of creating a
stable pH sensor which is re-usable and stable under
storage. In the course of the investigation and
optimization of the sensor system developed, aspects
of the fabrication process such as heat treatment, the
deposition of two layers of PAH/SiO
2
as thin film
‘topping’ layers and the deposition of two layers of
APTMS/SiO
2
as similar ‘topping’ layers have been
investigated and the resulting sensors characterized to
determine the best approach to creating a sensor
which is stable and reliable in operation: thus giving
the same calibration, in terms of the value of the peak
wavelength for a particular value of pH, and doing so
in a reproducible way. Further aspects which relate to
improved sensor performance, such as longer shelf-
life, better stability and sensor re-usability after
cleaning for a sensor prepared using the LbL
technique in this work are considered and reported,
showing the value of the approach taken in this
research.
2 MATERIALS AND METHODS
2.1 Chemicals
In order to create an effective optical pH sensor,
brilliant yellow (BY) was selected as the pH indicator
to be used, as discussed in prior work by the authors
(Raoufi et al., 2012b). This indicator dye was chosen
for its wavelength variation and ease of use and it was
cross-linked to poly(allylamine hydrochloride)
[PAH] with an average molecular weight (MW)
~15000, supplied by Sigma-Aldrich. PAH is a
positively charged molecule and was used as a
polycation. 3-Aminopropyl-trimethoxy silane
(APTMS) (99%) as a silane coupling agent and SM-
30 containing 30 wt% SiO
2
nanoparticles in H
2
O as a
strength enhancer were used as supplied by Sigma-
Aldrich. The structures of these molecules are shown
schematically in Figure 1.
EnhancedStabilityandRe-usabilityoftheOpticalSensorforpHMonitoringUsingaLayer-by-layerDepositionTechnique
157
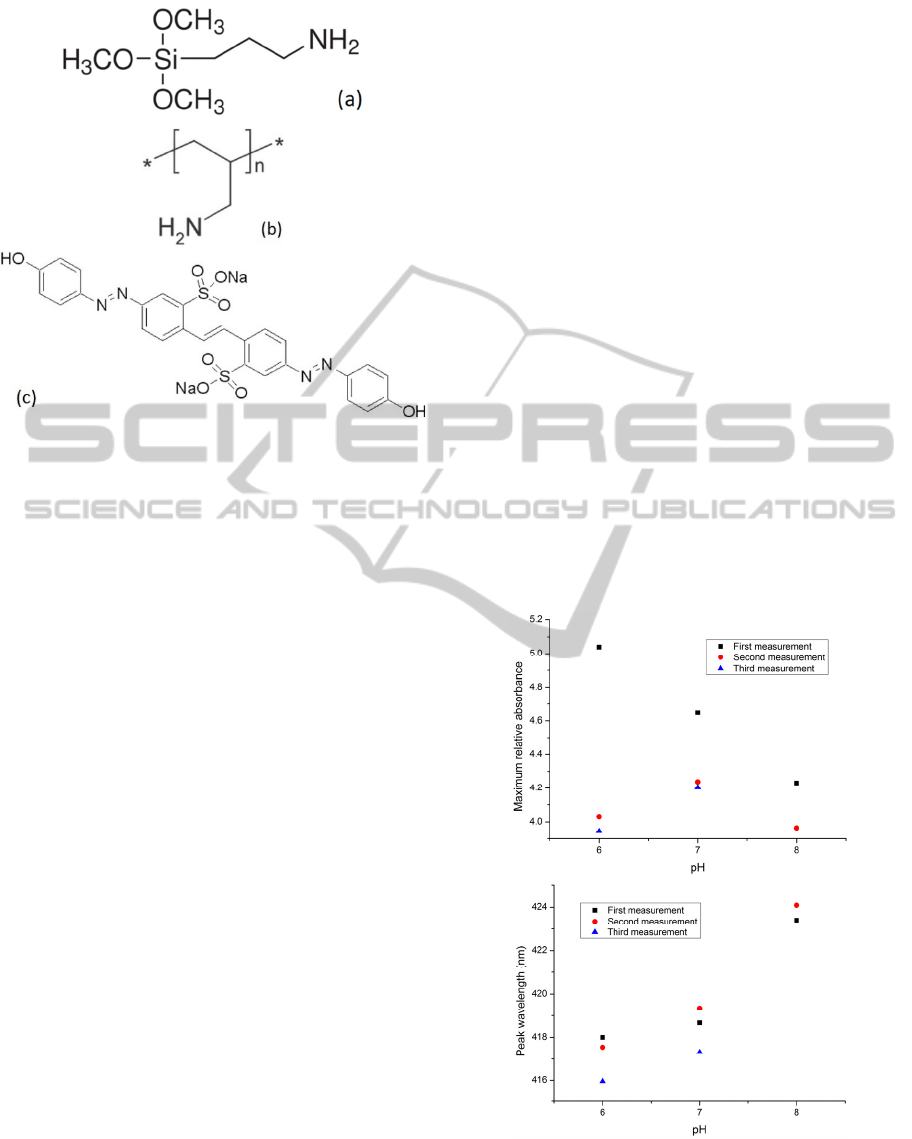
Figure 1: Chemical structure of (a) APTMS, (b) Poly
(allylamine) hydrochloride [PAH] and (c) brilliant yellow.
2.2 Procedures
The multilayer coating which was deposited by using
a self-assembly, Layer-by-Layer (LbL) technique
was carried out using a glass microscope slides of
dimensions 76×26 mm, with thickness of 1.0 mm.
The glass slide used was treated with Piranha solution
(30:70 (v/v) mixture of hydrogen peroxide (H
2
O
2
)
(30%) and concentrated sulfuric acid (H
2
SO
4
)) for 60
minutes to produce the negatively charged surface
and was then rinsed with distilled water, followed by
drying with compressed nitrogen. The glass slide was
then ready to be coated with positively charged
molecules. The Layer-by-Layer technique is based
on the successive deposition of oppositely charged
molecules onto a solid surface. In this technique, the
functionalized surface of a glass slide dipped into
PAH solution for 5 minutes to create a polycation
layer. The glass slide was then dipped into distilled
water for 5 minutes to wash off the un-bonded
molecules. This glass slide was then dipped in BY
solution for 5 minutes to construct the polyanion layer
above the PAH layer. To wash away the un-bonded
molecules, the glass slide was then immersed in fresh
distilled water for 5 minutes. This operation was then
repeated six times to build up six bilayers of
(PAH/BY) and thus increase the thickness of the thin
film deposited on the glass slide, which then was
ready to be used as the active element in the sensor
system, tested by being used for measurement of the
pH of a buffer solution. The performance of the
sensor prepared was examined through the
measurement and the evaluation of the change in the
peak wavelength of the absorption when the sensor
was evaluated by being dipped into buffer solutions
of different and varying pH. The process was carried
out by dipping the sensor slide into the pH solution
for a few minutes, following which the absorbed light
versus wavelengths is measured by use of the Perkin
Elmer spectrophotometer. The glass slide was then
taken out of the solution and was immersed in
another, fresh buffer solution (of different and known
pH), this being following by a measurement of the
absorbance spectra. The measurement was carried out
several times, using fresh buffer solutions increasing
from pH 6 to 9, and then decreasing from pH 9 to 6,
this being repeated typically two or three times for a
number of such samples. The maximum value of the
absorbance was normalized by dividing by the
minimum value and th results were plotted as a graph
of relative absorbance versus pH. For consistency, the
preparation conditions and the concentration of the
polyion solutions used were the same in all these
experiments. However, it was found that to achieve a
sensor performance that is consistent and shows the
same values of peak wavelength for an identical pH,
especially after -three times of use, further operations
on the design and construction of the sensor are
needed and this is discussed in the following work.
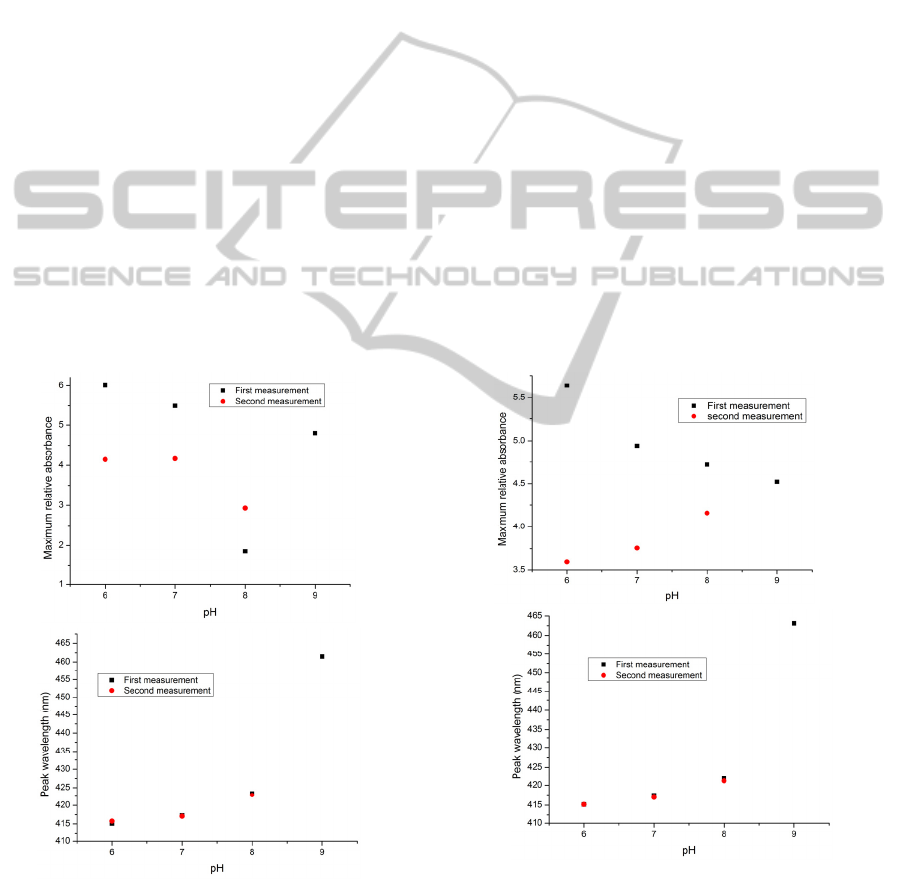
Figure 2: The maximum value of the relative absorbance
(up) and the peak wavelength (down) versus pH for the
consecutive measurements of the pH solutions for
(PAH/BY)
8
on the glass slide. No heat treatment or drying
was used in the process (The sample is designated GS01).
PHOTOPTICS2015-InternationalConferenceonPhotonics,OpticsandLaserTechnology
158
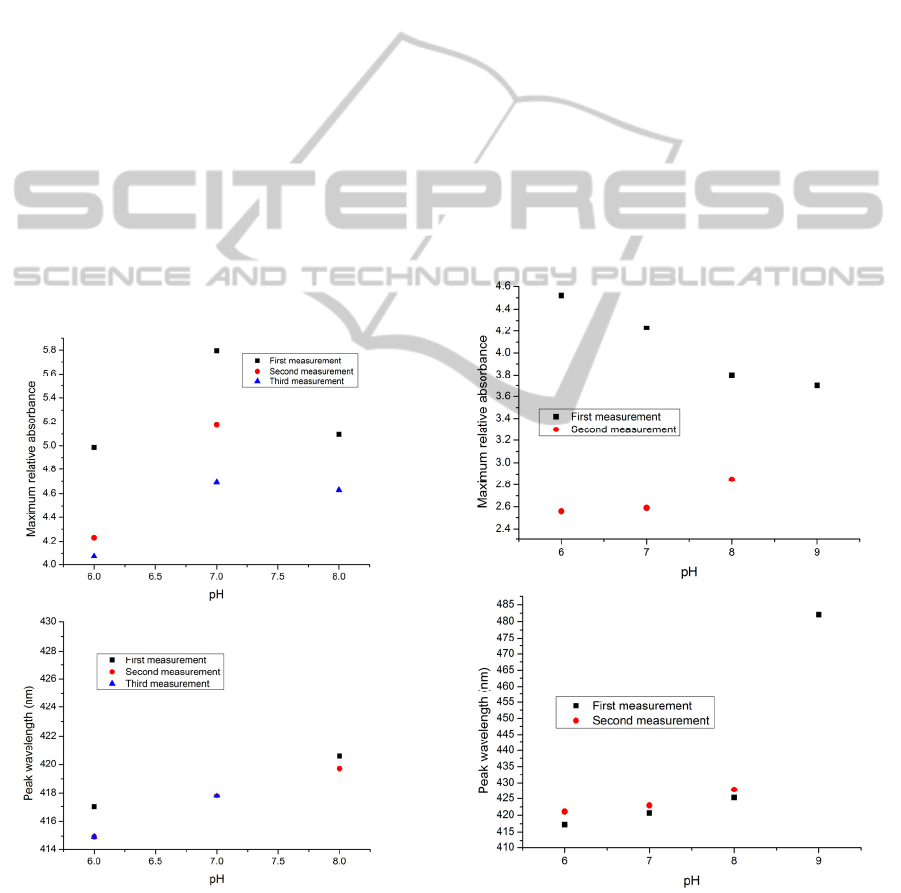
2.3 Experiments
Following the approach in previous research reported
by (Raoufi et al., 2012b, Raoufi et al., 2013), a glass
slide was prepared on which was deposited alternate
layers of brilliant yellow (BY) (as a pH indicator) and
poly (allylamine hydrochloride) [PAH] (as a cross-
linker of the layers of brilliant yellow), using layer-
by-layer coating technique. To determine the stability
of thin film, initially neither heat treatment or drying
was used in the process of coating of glass slide with
a total of 8 bilayers of (PAH/BY); this configuration
is denoted by (PAH/BY)
8
, where the subscript
indicates the number of bilayers, in this case 8. As
high pH can discharge the first polyion layer and
destroy the ionic bonds that stabilize the films, to
prevent this action solutions with a pH in the range
from 6 to 8 (over the neutral pH range) only were
examined. The results of several (three) successive
measurements using this sensor approach are shown
in Figure 2. However, it can be seen that the stability
of the bilayer system thus prepared could be enhanced
and this was achieved by further operations including
heat treatment or applying a capping layer. In order to
Figure 3: The maximum value of the relative absorbance
(up) and the peak wavelength (down) versus pH for the
consecutive measurements of the pH solutions for
(PAH/BY)
16
on the glass slide. The sample was cured at
120°C for 4 hours (The sample is designated GS02).
determine the most efficient method, a number of
different methods were considered and applied
including a) heat treatment b) covering the layers with
PAH and silica nanoparticles and c) coating the layers
with APTMS and silica nanoparticles as discussed
below.
2.3.1 Heat Treatment
In order to make a stable thin film, two glass slides
coated as indicated with (PAH/BY)
16
and (PAH/BY)
6
and cured in 120°C for 4 hours were examined.
The glass slide coated with (PAH/BY)
16
was
investigated using buffer solutions with pH varying
from 6 to 8 and then back from 8 to 6, while the other
slide was tested in a similar way with buffer solutions
ranging from 6 to 9. The maximum value of the
relative absorbance and the peak wavelength versus
pH for the consecutive measurements of the pH of the
solutions investigated for these two samples are
shown in Figure 3 and Figure 4.
Figure 4: The maximum value of the relative absorbance
(up) and the peak wavelength (down) versus pH for the
consecutive measurements of the pH solutions for
(PAH/BY)
6
on the glass slide. The sample was cured at
120°C for 4 hours (The sample is designated GS03).
EnhancedStabilityandRe-usabilityoftheOpticalSensorforpHMonitoringUsingaLayer-by-layerDepositionTechnique
159
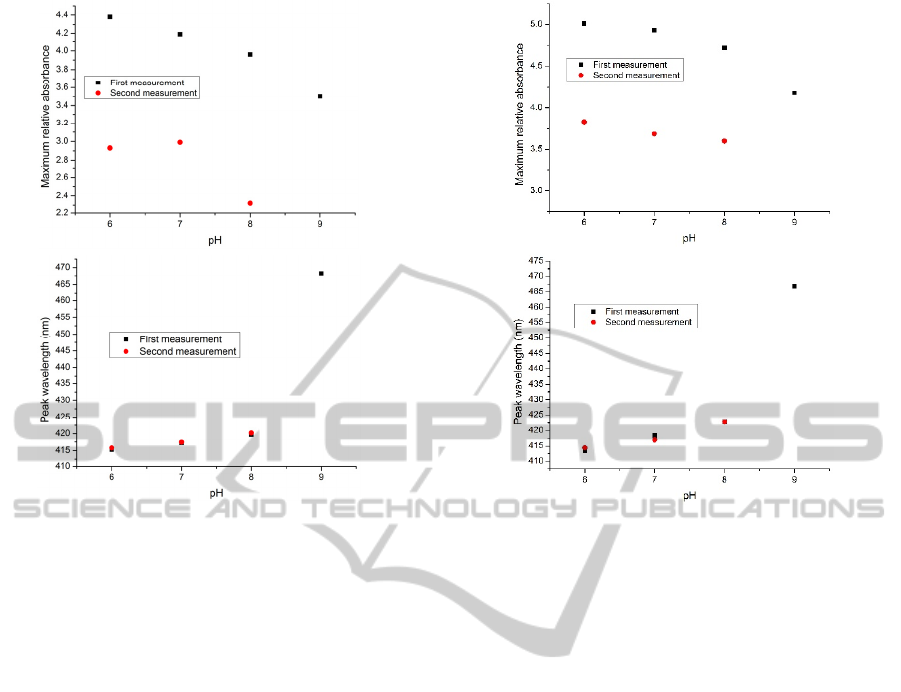
Figure 5: The maximum value of the relative absorbance
(up) and the peak wavelength (down) versus pH for the
consecutive measurements of the pH solutions for
(PAH/BY)
6
(PAH/SiO
2
)
2
on the glass slide (The sample
designated as GS04).
2.3.2 Using PAH and Silica Nanoparticle
Coatings
The use of silica nanoparticles covering the indicator
multilayers is designed to enhance the strength of the
film. Therefore, a series of experiments was carried
out and discussed below in which layers of silica
nanoparticles were built up on a glass slide already
coated with 6 bilayers of (PAH/BY), i.e (PAH/BY)
6
.
Different methods of curing were examined to find
out the most appropriate way to develop the most
stable coating. The silica solution used (SiO
2
) was
prepared at 1 wt.% (1.7mM) concentration. As the
SiO
2
solution is strongly alkaline (with pH 10.5), it
causes the destruction of the BY layers; hence the pH
of the SiO
2
solution was adjusted to pH 7 by adding
some drops of HCl to the solution before the
deposition of the layers was commenced. The glass
slide thus prepared with (PAH/BY)
6
was then
functionalized by using two bilayers of (PAH/SiO
2
)
followed by curing at 120°C for four hours (this
sample being designated GS04). The results of the
measurements taken using this sensor sample, over
the range from pH 6 to pH 9 and from 9 back to 6, is
shown in Figure 5. In a further experiment, the
temperature used for the annealing of the thin film
coating and comprising the following combination –
Figure 6: The maximum value of the relative absorbance
(up) and the peak wavelength (down) versus pH for the
consecutive measurements of the pH solutions for
(PAH/BY)
6
(PAH/SiO
2
)
2
on the glass slide. The sample was
cured at 130°C for 7 hours (The sample designated as
GS05).
(PAH/BY)
6
(PAH/SiO
2
)
2
was heat treated for a
longer period, of to 7 hours, at 130°C (the sample
being designated GS05) was and exposed to the
buffer solutions. Experiment has shown that higher
temperatures cannot be applied successfully because
of the degradation caused to the BY material.
In the next experiment, UV irradiation was utilized to
provide energy to cause the layers to form a stronger
bond, in addition to the electrostatic attraction
present. The silica nanoparticle coating is a
photosensitive material and the ionic bonds between
SiO
2
and PAH may be converted to covalent bonds
by use of this UV irradiation. To do so, the sample
was exposed to the UV light (irradiation intensity:
1112 mWcm
-2
at 365 nm) for 20 minutes, after
annealing at 130°C for 7 hours and then the sensor
was exposed to the different pH buffer solutions. The
results of the spectra recorded for this sample
(designated GS06) are shown in Figure 7. The silica
nanoparticles act as polyanions in the same way as the
BY material. It can be speculated that if the silica
molecules are located amongst the molecules of
brilliant yellow (BY), then the formation of the
molecular bonds between the silica and the PAH
molecules would create a bilayer which would
PHOTOPTICS2015-InternationalConferenceonPhotonics,OpticsandLaserTechnology
160
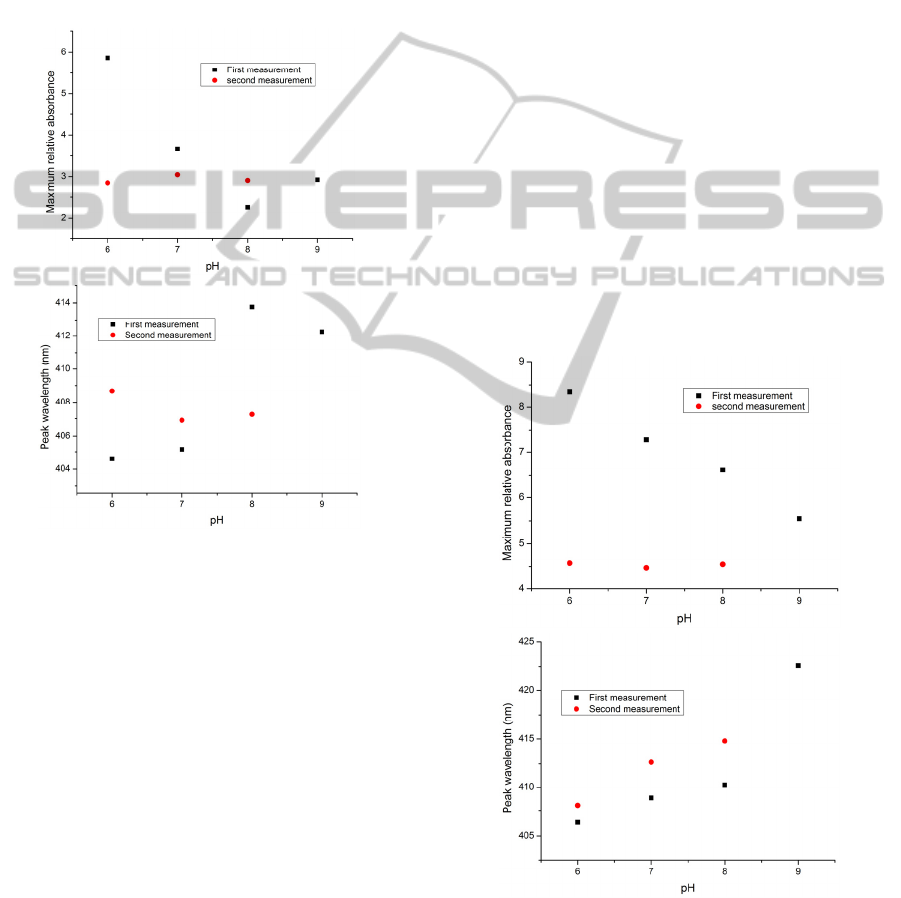
surround the BY molecules in each layer. Therefore,
a further set of experiments was carried out in which
a silica nanoparticle solution was added to a BY
solution (1.7 mM SiO
2
: 0.25 mM BY) and the pH of
the solution was adjusted to pH 6. Having coated the
glass slide sensor in this way to create
(PAH/(BY+SiO
2
))
6
, then annealing at 120°C for 4
hours, the deposited glass slide (designated GS07)
was examined under various buffer solutions of
different values of pH. The results of this test and
thus the recorded spectra are shown in Figure 8.
Figure 7: The maximum value of the relative absorbance
(up) and the peak wavelength (down) versus pH for the
consecutive measurements of the pH solutions for
(PAH/BY)
6
(PAH/SiO
2
)
2
on the glass slide. The sample was
cured at 130°C for 7 hours and exposed to UV light for 20
minutes (The sample designated as GS06).
2.3.3 Use of APTMS and Silica
Nanoparticles
Further experimentation was carried out by replacing
the polycation; PAH; with 3-Aminopropyl-
trimethoxysilane (APTMS) in the capping layers.
APTMS is known as a silane coupling agent in which
a water based solution is polymerized after
hydrolysis, as shown schematically in Figure 9. The
surface of the SiO2 nanoparticle thin film contains
hydroxyl groups in the form of SiOH. These groups
may donate or accept a proton from the solution,
leaving a negativelycharged or a positively charged
surface group respectively (Liu and Cui, 2007).
Therefore, there are two possibilities that the
molecules of APTMS bond to the silica nanoparticles;
the molecule constitutes of an amine group as a
positive charge supplier and hydroxyl groups as
negative charges supplier which bond to silicon, as
shown in Figure 10. In the experiment, the solution
of 1 wt.% APTMS in H
2
O (1.4 mM) used was
strongly alkaline (with pH 10.8) and should thus be
adjusted to the neutral pH (~pH 6) that is best suited
for use as polyelectrolyte in the LbL technique which
is possible by adding several drops of HCl to both
solutions of the polycation and polyanion.
Two glass slide sensor samples coated with
(PAH/BY)
6
(APTMS/SiO
2
)
2
were prepared; one of
them was examined the same day of preparation (the
sample designated GS08) and the second (the sample
designated GS09) was evaluated a week later. The
results of the tests carried out are shown in Figure 11
and Figure 12 respectively.
To investigate the effect of the use of APTMS alone,
a thin film comprising 6 bilayers of (PAH/BY),
dipped in APTMS solution for 30 minutes, followed
by dipping in distilled water for 5 minutes, then cured
at 120°C for 4 hours was examined. The results of
this experiment is shown in Figure 13.
Figure 8: The maximum value of the relative absorbance
(up) and the peak wavelength (down) versus pH for the
consecutive measurements of the pH solutions for
(PAH/(BY+SiO
2
))
6
on the glass slide. The sample was
cured at 120°C for 4 hours (sample designated GS07).
EnhancedStabilityandRe-usabilityoftheOpticalSensorforpHMonitoringUsingaLayer-by-layerDepositionTechnique
161
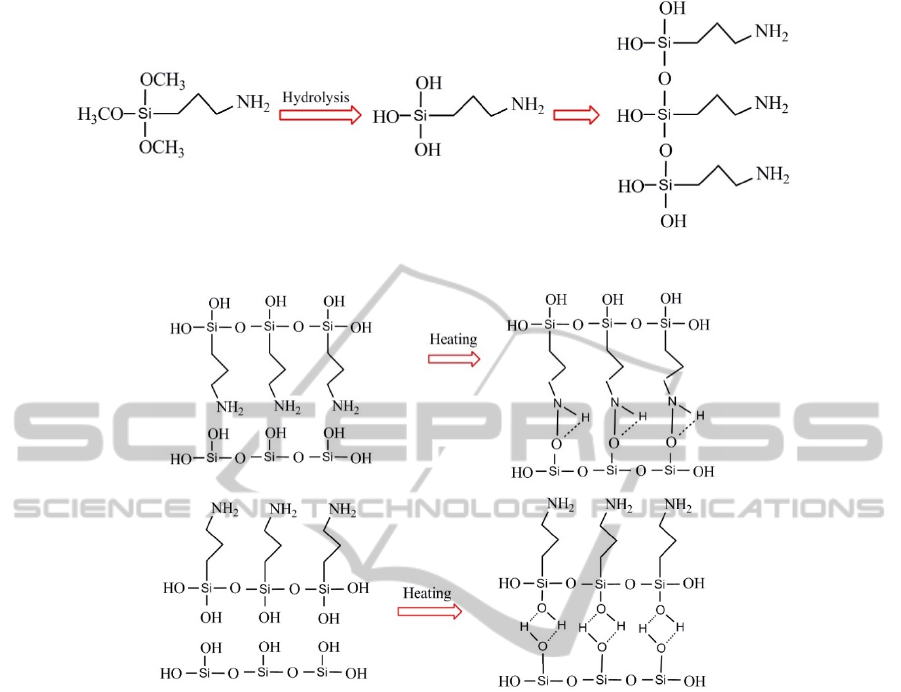
Figure 9: APTMS is polymerized in aqueous solution after hydrolysis.
Figure 10: Two possibilities reaction between molecules of APTMS and silica nanoparticles.
2.3.4 Re-usability and Aging
To investigate the effect on sensor stability of the
storage of the glass slides, different coated glass
slides of different types were examined. Thus in this
investigation, initially the glass slide with a coating
was dipped into a fresh buffer solution with constant
pH for 60 minutes and after this time the absorbance
spectra was recorded as a reference. After that the
glass slide was removed from that solution and then
was immersed into another solution (with the same
pH) for a further 60 minutes and a second absorbance
spectra was recorded. This operation was repeated a
third time. Several different slides with different films
were recorded: the stability and thus the re-usability
of the multilayer thin film slides using two different
samples with different structures, these being
(PAH/BY)
6
and (PAH/BY)
6
(APTMS/SiO
2
)
2
were
examined at two different values of pH; pH 6 and 8,
separately. The results of this test are shown in Figure
14 and Figure 15 and the positive results obtained
imply the stability of these two sensor samples under
exposure to these solutions for the times indicated.
3 RESULTS AND DISCUSSION
In the layer-by-layer coating technique, the stability
of the layers is seen to depend on type and
concentration of the salt, the strength of ions and
polyelectrolytes, the polymer molecular weight, the
pH of the solutions and the thermal energy. In order
to form a stable multilayer thin film, a minimum
charge density in each layer is needed which depends
on the salt concentration and the salt type; this was
investigated (Raoufi et al., 2012a) by the authors
previously and also it was shown that to rely very
strongly on the chemical identity of the charged units
involved in forming the thin film (Bertrand et al.,
2000). Stronger ion pairing will also yield more stable
multilayers (Decher and Schlenoff, 2002) and
moreover, high molecular weight polymers promote
PHOTOPTICS2015-InternationalConferenceonPhotonics,OpticsandLaserTechnology
162
the stability of the layers (Shimazaki et al., 2001,
Tang et al., 2002, Kotov et al., 1998). High and low
pH solutions can potentially discharge the ions and
destroy the layers, while heat treatment causes a
chemical reaction between the molecules of two
adjacent layers and makes the bonds stronger and
creates a more stable multilayer film, as can be seen
in the first three experiments discussed (shown in
Figure 2, Figure 3 and Figure 4). As the figures show,
in this series of experiments there is a continuous
decrease in the maximum absorbance for each pH
buffer solution for two successive measurements
while the values of the peak wavelength show a
significant change from the first to the second
measurement for the sample without heat treatment,
and this represents an increase in stability of the thin
film because of the thermal effect. In the sample
designated GS03 and examined in solutions ranging
from pH 6 to pH 9, a dramatic decline in the
maximum absorbance was seen, compared to other
samples examined over the range from pH 6 to pH 8.
The reduction in absorbance is a clear proof that
leaching of the indicator is occurring and the decrease
of the thickness of the thin film. In particular, in the
case where a pH 9 solution is used, which causes
greater destruction of the layers, a noticeable decrease
Figure 11: The maximum value of the relative absorbance
(up) and the peak wavelength (down) versus pH for the
consecutive measurements of the pH solutions for
(PAH/BY)
6
(APTMS/SiO
2
)
2
on the glass slide. The sample
was cured at 120°C for 4 hours (The sample designated
GS08).
takes place in the absorbance from the first to the
second measurement. In addition to the absorbance,
in all the samples studied the wavelength has not
stayed stable during consecutive measurements and a
small change of around 0 to 2 nm in the peak
wavelength was observed; although with increasing
the number of bilayers the amount of this change is
reduced. Covering the sensitive thin film with a
couple of layers of silica nanoparticles leads to a
coating which creates such a stable film that the
wavelength continues to remain constant for a certain
known pH, while the absorbance decreases
irregularly during the two cycles of measurement, as
shown in Figure 5. Referring to Figure 6, the duration
and the temperature of thermal treatment used did not
have any significant effect on the stability of the film
as the values of the peak wavelengths for the first and
the second measurements for both samples used,
designated GS04 and GS05, were roughly the same.
However, exposing the samples to UV light did not
modify the molecular bonds. As Figure 7 shows there
is not a regular change in the peak wavelength but a
drastic decline is seen in both the absorbance and the
peak wavelength values compared to previous
experiments and also a dramatic absorbance
reduction was observed in the first measurement.
Figure 12: The maximum value of the relative absorbance
(up) and the peak wavelength (down) versus pH for the
consecutive measurements of the pH solutions for
(PAH/BY)
6
(APTMS/SiO
2
)
2
on the glass slide. The sample
was cured at 120°C for 4 hours and examined after a week
(The sample designated GS09).
EnhancedStabilityandRe-usabilityoftheOpticalSensorforpHMonitoringUsingaLayer-by-layerDepositionTechnique
163
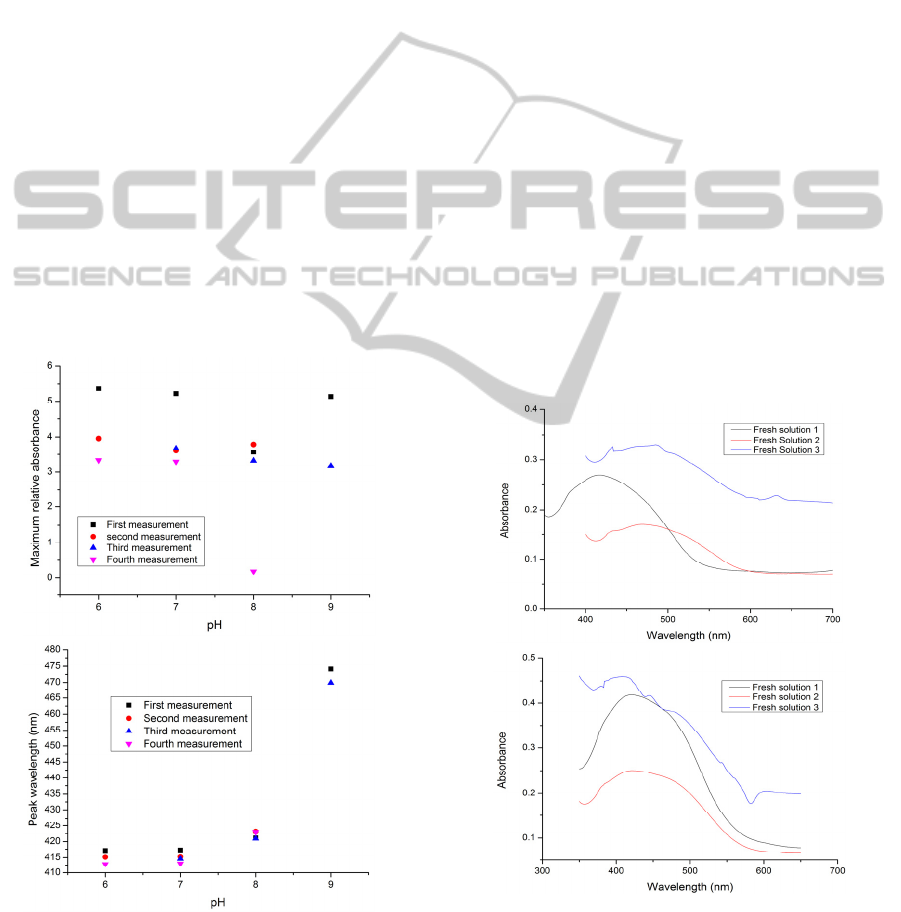
This means that by applying UV irritation, an increase
in the strength of the inter-molecular bonds occurs
temporarily.
However, these inter-molecular forces are loss after a
few times of dipping the sensor slide into the buffer
solutions. As a result, the molecules of BY start to
leach out from the sensitive thin film and this causes
a steep decrease in absorbance and a very undesirable
situation for a sensor system.
Adding silica nanoparticles to BY solutions shifts the
absorbance spectra to the lower peak wavelength
values, as shown in Figure 8. It can be seen in this
figure that the absorbance has increased significantly
compared to that of the other samples; however, the
values of both the absorbance and the peak
wavelength in the second measurement become close
to the values seen from the previous tests. Looking
more closely, it can be said that the higher values of
maximum absorbance in the first measurement imply
a larger number of molecules of brilliant yellow in the
deposited layers. In other words, the SiO
2
-PAH
bilayers can protect the BY from significant damage,
but not for a long time.
When the PAH was replaced by APTMS in the
capping layers, the results showed that the peak
Figure 13: The maximum value of the relative absorbance
(up) and the peak wavelength (down) versus pH for the
consecutive measurements of the pH solutions for
(PAH/BY)
6
APTMS on the glass slide. The sample was
cured at 120°C for 4 hours (The sample designated GS10).
wavelength for each pH solution used demonstrated
almost same values as shown in Figure 12. Table
1compares the peak wavelengths of three slides
(designated GS04, GS08 and GS09) for two
successive measurements over the range from pH 6 to
pH 9 and then from 9 to 6. It is noticeable that the
APTMS does not cause any significant changes to the
peak wavelengths, compared to the use of PAH and
the three tests carried out exhibited the same peak
wavelengths, in spite of the difference in the peak
intensity. The most striking result of this series of
experiments is the remarkable reduction of the
degradation of the probes and thus the improvement
that would be seen to their shelf-life, as well as their
re-usability, which are key considerations for the use
of this type of coating in an industrial sensor system.
Figure 13 shows that when the sensitive film is coated
with a layer of APTMS, the peak wavelength for each
pH value changes during successive measurements
and also there is not a regular shift with different
times of measurements. This therefore implies that
the stability of the measured wavelength cannot be
maintained because of the polycation properties.
Moreover, the influence of SiO
2
is more effective in
creating a stable sensor than does the polycation.
However, the cross-comparison of the two sensor
samples (designated as GS03 (without APTMS) and
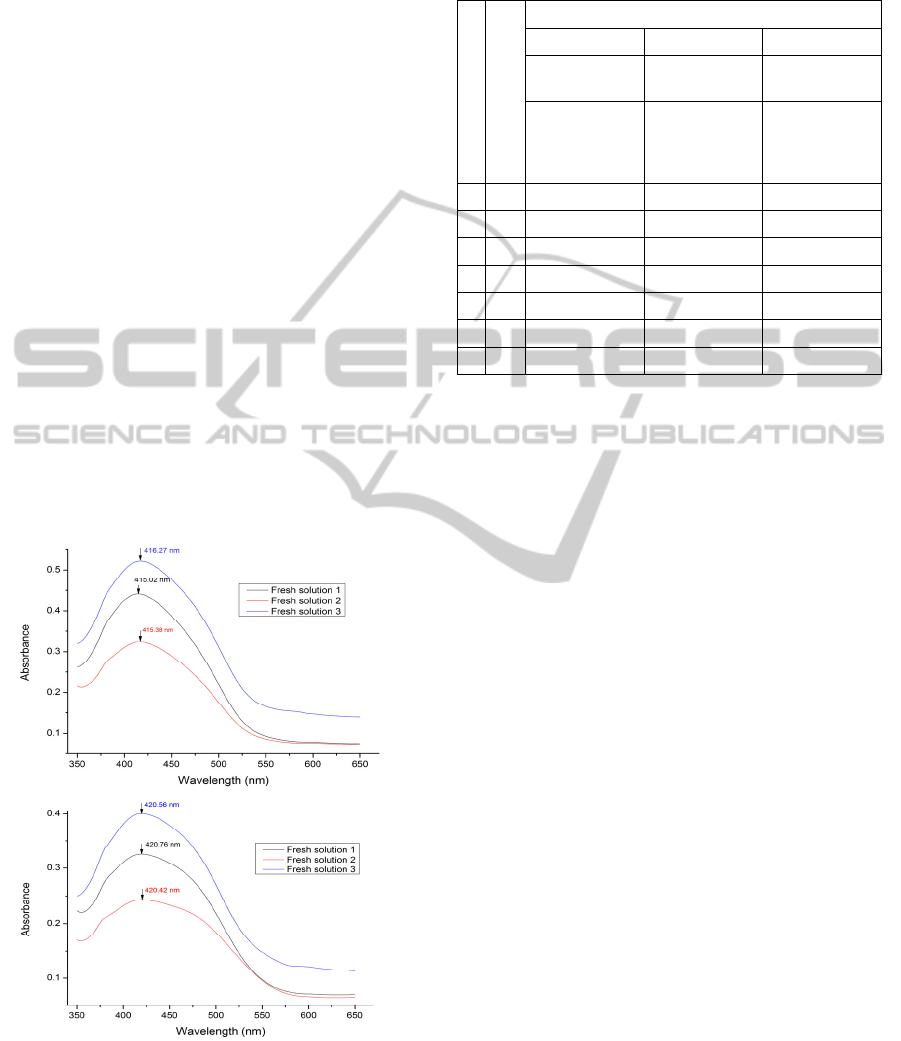
Figure 14: The spectra for the coated glass slides with
(PAH/BY)
6
dipped into the fresh buffer solution for three
times and each time for 60 minutes. Up: pH 6 (The sample
designated GS11), Down: pH 8 (The sample is designated
GS12). The samples were cured at 120°C for 4 hours.
PHOTOPTICS2015-InternationalConferenceonPhotonics,OpticsandLaserTechnology
164
GS10 (with a layer of APTMS)) and shown in Figure
4 and Figure 13 confirms that the effect of APTMS
on stability cannot be ignored. Indeed, as Table 1
shows, there is no significant difference in the
stability observed between the situations where two
capping layers of (PAH/SiO
2
) and (APTMS/SiO
2
) are
applied. This may arise because of the similarity
between the functional groups (amine groups) in both
the PAH and the APTMS and thus the same
interaction occurring between the polyanion and the
polycation species.
In addition, a comparison of the last two columns of
Table 1 shows that storing the samples for a week has
had a negligible effect on the peak wavelength values.
Applying these capping layers not only improved the
sensor shelf-life but also enhances the re-usability of
the sensor, as can be seen when sensors designated
GS11 and GS12 are compared to GS13 and GS14, as
illustrated in Figure 14 and Figure 15, respectively.
Figure 14 shows a result which implies that destroying
the deposited layers after 60 minutes exposure to the
buffer solution results in the peak wavelength staying
constant for those samples covered with two layers of
APTMS/SiO
2
, even after immersion on two separate
occasions in the buffer solutions, for periods of 60
minutes in each case.
Figure 15: The spectra for the coated glass slides with
(PAH/BY)
6
(APTMS/SiO
2
)
2
dipped into the fresh buffer
solution for three times and each time for 60 minutes. Up:
pH 6 (The sample is designated GS13), Down: pH 8 (The
sample is designated GS14). The samples were cured at
120°C for 4 hours.
Table 1: The peak wavelength for two times consecutive
measurements for the glass slides, designated GS04, GS08
and GS09 in pH solutions from 6 to 9 and then from 9 to 6.
p
H
Measurement
Peak wavelength (nm)
GS04 GS08 GS09
(PAH/BY)
6
(PA
H/SiO
2
)
2
(PAH/BY)
6
(AP
TMS/SiO
2
)
2
(PAH/BY)
6
(AP
TMS/SiO
2
)
2
Error (%)
Error (%)
Error (%)
6
1
S
t
415.26 - 415.10 - 415.18 -
7
1
S
t
417.24 - 417.26 - 417.36 -
8
1
S
t
423.19 - 423.4 - 421.92 -
9
1
S
t
468.25 - 461.5 - 463.12 -
8
2
nd
423.09 -0.02 422.9 -0.1 421.17 -0.2
7
2
nd
417.45 0.05 417.06 -0.08 417.06 -0.07
6
2
nd
415.75 0.1 415.75 0.1 415.18 0
4 CONCLUSIONS
In this study, the stability and the deterioration of
wavelength-dependent optical sensor devices to pH
changes using techniques to build up a series of
nanolayer coatings was investigated and the results
reported.
The stability of the pH sensors thus fabricated was
studied using different pH buffer solutions. A variety
of techniques was proposed and investigated to
improve the stability of the film and to avoid
progressive destruction of the indicator layer. One
technique explored extensively was to build up
capping bilayers using different materials i.e. silica
nanoparticles as the polyanion and PAH or APTMS
as the polycation. Another approach investigated was
to apply thermal treatment after the layer building
process and an alternative method considered was
applying UV light irradiation.
The results of the experiments carried out,
including covering the sensitive thin film with two
layers of (APTMS/silica) nanoparticles was shown to
improve the stability of the sensor (indicated by the
stability of the peak wavelength) and also to enhances
the stability and potential shelf life of the sample,
when the nanolayers are deposited on the glass slide
substrates. The most striking result is that the peak
wavelength continues to remain constant for a
particular pH while the layers were affected during
repeated measurements over three cycles.
The durability of the sensors thus created makes
the probe a suitable wavelength-dependent
EnhancedStabilityandRe-usabilityoftheOpticalSensorforpHMonitoringUsingaLayer-by-layerDepositionTechnique
165

measurement device which is well suited to use as a
high resolution pH sensor. To do so, the layers
described can be coated directly on optical fibres of
various diameters or coated glass substrates can be
attached to the distal end of the fibre probes created.
In that way compact optical fibre sensors can be
created for a range of applications and as the work has
shown, stored for some time before use.
ACKNOWLEDGMENTS
This work was supported by Islamic Azad University-
South Tehran Branch and City University London.
REFERENCES
Ai, J., Jones, S. A. and Lvov, Y. M. 2003. Biomedical
Applications of Electrostatic Layer-By-Layer Nano-
Assembly of Polymers, Enzymes, and Nanoparticles.
Cell Biochemistry and Biophysics, 39, 23-43.
Bertrand, P., Jonas, A., Laschewsky, A. and Legras, R.
2000. Ultrathin Polymer Coatings by Complexation of
Polyelectrolytes at Interfaces: Suitable Materials,
Structure and Properties. Macromolecular Rapid
Communications, 21, 319-348.
Cassagneau, T., Mallouk, T. E. and Fendler, J. H. 1998.
Layer-by-layer Assembly of Thin Film Zener Diodes
from Conducting Polymers and Cdse Nanoparticles.
Journal Of The American Chemical Society, 120, 7848-
7859.
De Villiers, M. M., Otto, D. P., Strydom, S. J. and Lvov, Y.
M. 2011. Introduction to Nanocoatings Produced by
Layer-by-layer (Lbl) Self-Assembly. Advanced Drug
Delivery Reviews, 63, 701-715.
Decher, G., Hong, J. D. and Schmitt, J. 1992. Buildup Of
Ultra Thin Multi Layer Films By A Self Assembly
Process: Ii. Consecutively Alternating Adsorption Of
Anionic And Cationic Poly Electrolytes On Charged
Surfaces. Thin Solid Films, 831, 210-211.
Decher, G., Lvov, Y. and Schmitt, J. 1994. Proof Of
Multilayer Structural Organization In Self-Assembled
Polycation-Polyanion Molecular Films. Thin Solid
Films, 244, 772-777.
Decher, G. and Schlenoff, J. B. 2002. Multilayer Thin
Films, Wiley-Vch Verlag Gmbh and Co.
Dubas, S. T., Kumlangdudsana, P. and Potiyaraj, P. 2006.
Layer-by-layer Deposition of Antimicrobial Silver
Nanoparticles on Textile Fibers. Colloids and Surfaces
A: Physicochemical and Engineering Aspects, 289,
105-109.
Egawa, Y., Hayashida, R. and Anzai, J.-I. 2007. Covalently
Cross-Linked Multilayer Thin Films Composed of
Diazoresin And Brilliant Yellow for an Optical Ph
Sensor Polymer, 48, 1455-1458.
Ichinose, I., Muzuki, S., Ohno, S., Shiraishi, H. and
Kunitake, T. 1999. Preparation of Cross-Linked
Ultrathin Films Based on Layer-by-layer Assembly of
Polymers. Polymer Journal, 31, 1065-1070.
Johnston, A. P. R., Cortez, C., Angelatos, A. S. and Caruso,
F. 2006. Layer-By-Layer Engineered Capsules and
Their Applications. Current Opinion In Colloid and
Interface Science, 11, 203-209.
Kotov, N. A., Magonov, S. and Tropsha, E. 1998. Layer-
By-Layer Self-Assembly of Alumosilicate−
Polyelectrolyte Composites: Mechanism of
Deposition, Crack Resistance, and Perspectives for
Novel Membrane Materials. Chemistry of Materials,
10, 886-895.
Lee, D. and Cui, T. 2012 A Role of Silica Nanoparticles In
Layer-by-layer Self-Assembled Carbon Nanotube and
In2o3 Nanoparticle Thin-Film Ph Sensors: Tunable
Sensitivity And Linearity. Sensors and Actuators A:
Physical, 188, 203-211.
Li, Q., Quinn, J. F. and Caruso, F. 2005. Nanoporous
Polymer Thin Films Via Polyelectrolyte Templating.
Advanced Materials, 17, 2058-2062.
Liu, Y. and Cui, T. 2007. Ion-Sensitive Field-Effect
Transistor Based Ph Sensors Using Nano Self-
Assembled Polyelectrolyte/Nanoparticle Multilayer
Films. Sensors And Actuators B 123, 148-152.
Llobet, E. 2013. Gas Sensors Using Carbon Nanomaterials:
A Review. Sensors And Actuators B 179, 32- 45.
Nallathambi, G., Ramachandran, T., Rajendran, V. and
Palanivelu, R. 2011. Effect of Silica Nanoparticles and
Btca on Physical Properties of Cotton Fabrics.
Materials Research, 14, 552-559.
Potapov, V. V., Shitikov, E. S., Trutnev, N. S., Gorbach, V.
A. and Portnyagin, N. N. 2011. Influence of Silica
Nanoparticles on the Strength Characteristics of
Cement Samples. Glass Physics And Chemistry, 37, 98-
105.
Prakash, S., Chakrabarty, T., Singh, A. K. and Shahi, V. K.
2013. Polymer Thin Films Embedded With Metal
Nanoparticles For Electrochemical Biosensors
Applications. Biosensors And Bioelectronics, 41, 43-
53.
Putzbach, W. and Ronkainen, N. 2013. Immobilization
Techniques In The Fabrication Of Nanomaterial-Based
Electrochemical Biosensors: A Review. Sensors, 13,
4811-4840.
Rahman, I. A. and Padavettan, V. 2012. Synthesis of Silica
Nanoparticles by Sol-Gel: Size-Dependent Properties,
Surface Modification, and Applications In Silica-
Polymer Nanocomposites—A Review. Journal of
Nanomaterials, 2012, 1-15.
Raoufi, N., Surre, F., Rajarajan, M., Sun, T. and Grattan, K.
2013. Fibre Optic Ph Sensor Using Optimized Layer-
By-Layer Coating Approach Sensors Journal, Ieee In
Press.
Raoufi, N., Surre, F., Sun, T., Grattan, K. T. V. and
Rajarajan, M. Improvement of Optical Properties of Ph-
Sensitive Nanolayers Coating Deposited Using Layer-
by-layer Technique. Sensors, 2012 Ieee, 28-31 Oct.
2012 2012a. 1-4.
PHOTOPTICS2015-InternationalConferenceonPhotonics,OpticsandLaserTechnology
166

Raoufi, N., Surre, F., Sun, T., Rajarajan, M. and Grattan, K.
T. V. 2012b. Wavelength Dependent Ph Optical Sensor
Using The Layer-By-Layer Technique. Sensors And
Actuators B: Chemical, 169, 374-381.
Saeki, D., Imanishi, M., Ohmukai, Y., Maruyama, T. and
Matsuyama, H. 2013. Stabilization Of Layer-By-Layer
Assembled Nanofiltration Membranes By Crosslinking
Via Amide Bond Formation And Siloxane Bond
Formation. Journal Of Membrane Science, 447, 128-
133.
Sen, A. K., Mukherjee, B., Bhattacharyya, A. S., De, P. P.
and Bhowmick, A. K. 1992. Kinetics Of Silane
Grafting And Moisture Crosslinking Of Polyethylene
And Ethylene Propylene Rubber. Journal of Applied
Polymer Science, 44, 1153-1164.
Sharma, R. K., Chan, P. C. H., Tang, Z., Yan, G., Hsing, I.
M. and Sin, J. K. O. 2001. Sensitive, Selective And
Stable Tin Dioxide Thin-Films For Carbon Monoxide
And Hydrogen Sensing In Integrated Gas Sensor Array
Applications. Sensors And Actuators B: Chemical, 72,
160-166.
Shimazaki, Y., Nakamura, R., Ito, S. and Yamamoto, M.
2001. Molecular Weight Dependence of Alternate
Adsorption Through Charge-Transfer Interaction.
Langmuir, 17, 953-956.
Singh, R., Goel, T. C. and Chandra, S. 2008. Rf Magnetron
Sputtered La3+-Modified Pzt Thin Films: Perovskite
Phase Stabilization And Properties. Materials
Chemistry And Physics, 110, 120-127.
Su, Y.-L. and Li, C. 2008. Stable Multilayer Thin Films
Composed Of Gold Nanoparticles And Lysozyme.
Applied Surface Science, 254, 2003-2008.
Tang, Z., Wang, Y. and Kotov, N. A. 2002. Semiconductor
Nanoparticles On Solid Substrates: Film Structure,
Intermolecular Interactions, And Polyelectrolyte
Effects. Langmuir, 18, 7035-7040.
Villar, I. D., Matias, I. R. and Arregui, F. J. 2008. Fiber-
Optic Chemical Nanosensors By Electrostatic
Molecular Self-Assembly. Current Analytical
Chemistry, 4, 341-355.
Villar, I. D., Matías, I. R., Arregui, F. J. and Claus, R. O.
2005. Esa-Based In Fiber Nanocavity For Hydrogen
Peroxide Detection. Ieee Transactions On
Nanotechnology, 4, 187-193.
Wang, D., Shakeel, H., Lovette, J., Rice, G. W., Heflin, J.
R. and Agah, M. 2013. Highly Stable Surface
Functionalization Of Microgas Chromatography
Columns Using Layer-By-Layer Self-Assembly Of
Silica Nanoparticles. Analytical Chemistry, 85, 8135-
8141.
Yamada, H., Imoto, T., Fujita, K., Okazaki, K. and
Motomura, M. 1981. Selective Modification Of
Aspartic Acid-101 In Lysozyme By Carbodiimide
Reaction. Biochemistry, 20, 4836-4842.
EnhancedStabilityandRe-usabilityoftheOpticalSensorforpHMonitoringUsingaLayer-by-layerDepositionTechnique
167
