Acute Changes in Carotid Arterial Blood Velocity Immediately after
the Cessation of Exercise
Midori Tanaka
1
, Motoaki Sugawara
1
, Yasuo Ogasawara
2
, Kiyomi Niki
3
and Tadafumi Izumi
4
1
Faculty of Health Care Sciences, Himeji Dokkyo University, Himeji, Japan
2
Department of Medical Engineering, Kawasaki Medical School, Kurashiki, Japan
3
Biomedical Engineering Department, Tokyo City University, Tokyo, Japan
4
School of Rehabilitation Sciences, Health Sciences University of Hokkaido, Tobetsu, Hokkaido, Japan
1 OBJECTIVES
Changes in hemodynamic parameters following
exercise have been widely reported. However, the
changes in carotid arterial blood flow have not been
well documented. The purpose of this study was to
examine acute changes in carotid arterial blood
velocity immediately after the cessation of exercise.
2 METHODS
Twenty-four young healthy men were registered
(age: 21.3 ± 1.6 years). The cardiopulmonary
exercise test was conducted starting at an initial
workload of 20 W and lasting for 2 minutes with a
strength ergometer; thereafter, the workload was
increased stepwise by 20 W at 1-minute intervals.
Electrocardiogram was continuously monitored. The
criteria for the endpoint included increase of heart
rate to [(220-age) ×0.8 (bpm)], and achievement of
maximum fatigue or the impossibility of continuing
exercise. Then cooling down was conducted at 20 W
following exercise for 150s. We measured the blood
velocity in the carotid artery at 60s and 120s after
the cessation of exercise with a Doppler system. We
also measured carotid arterial diameter by echo
tracking (Niki, 2002). Carotid arterial stroke volume
(FV) was obtained by integrating the product of
blood velocity and arterial cross-section over systole.
Carotid arterial output (Q) was given as FV times
heart rate. The data were analyzed by the repeated
measures ANOVA and Bonferroni post test.
3 RESULTS
Reductions in systolic/ diastolic blood pressure were
significant at 60s and 120s after the cessation of
exercise (93 ± 3 / 84 ± 3% , 88 ± 4 / 84 ± 3%,
respectively) compared with the values at the
cessation of exercise (100%). The reduction in heart
rate was also significant at 60s and 120s after the
cessation of exercise (84 ± 7% and 78 ± 7%,
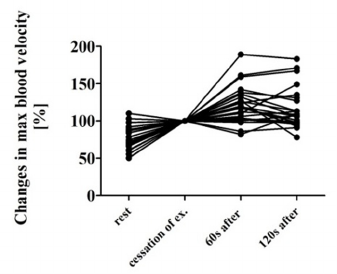
respectively). On the contrary, the maximum blood
velocity increased significantly to 121 ± 24 % and
116 ± 27% at 60s and 120s after the cessation of
exercise, respectively (Fig. 1). FV also increased
significantly to 128±40% and 129±42%. However,
Q remained unchanged.
Figure 1: The maximum carotid arterial blood velocities at
rest, at the cessation of exercise, 60s and 120s after the
cessation of exercise. Data are displayed as percent,
normalized to the velocity at the cessation of exercise.
4 DISCUSSION
Following exercise, syncope is often reported
(DiVasta, 2004; Natarajan, 2006; Vettor, 2015).
However, its hemodynamic mechanism is not yet
fully understood. The responses in blood flow (or
velocity) following exercise are not well
documented even though there has been extensive
work done on the blood pressure and heart rate
responses. The blood pressure and heart rate started
to decrease immediately after the cessation of
Tanaka, M., Sugawara, M., Ogasawara, Y., Niki, K. and Izumi, T..
Acute Changes in Carotid Arterial Blood Velocity Immediately after the Cessation of Exercise.
Copyright
c
2015 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

exercise as reported so far
(Muendel, 2015). On the
other hand, carotid arterial blood velocity increased
further after the cessation of exercise. Carotid
arterial stroke volume (FV) also increased, which
compensated for the reduction in output due to the
decrease in heart rate. Thus, carotid arterial output
(Q) remained unchanged. We consider that this is a
mechanism to prevent hemodynamic disturbance
after cessation of exercise.
5 CONCLUSIONS
Immediately after the cessation of exercise, blood
pressure and heart rate decreased. However, carotid
arterial blood velocity and stroke volume increased
significantly. As a result carotid arterial output
remained unchanged. This is considered to be a
mechanism to prevent post-exercise hemodynamic
disturbances.
REFERENCES
Niki, K., Sugawara, M., Chang, D., et al., 2002. A new
noninvasive measurement system for wave intensity:
evaluation of carotid arterial wave intensity and
reproducibility. Heart Vessels; 17: 12-21
DiVasta, A. D., Alexander M.E., 2004. Fainting freshmen
and sinking sophomores: cardiovascular issues of the
adolescent. Curr Opin Pediatr.;16(4):350-6.
Natarajan, B., Nikore, V. 2006. Syncope and near syncope
in competitive athletes. Curr Sports Med Rep;
5(6):300-6.
Vettor, G., Zorzi, A., Basso, C , 2015. Syncope as a
Warning Symptom of Sudden Cardiac Death in
Athletes. Cardiol Clin; 33(3):423-32. doi:
10.1016/j.ccl.2015.04.010.
Muendel, T., Perry, B.G., Ainslie, P.N., et al., 2015. Post-
exercise orthostatic intolerance: Influence of exercise
intensity. Exp Physiol; 100(8):915-25. doi: 10.
1113/EP085143. [Epub ahead of print].
