
Modeling the G-Protein Signaling of the Retina with Fractional Calculus
Antal Martinecz and Mihoko Niitsuma
Precision Mechanics, Chuo University, 1-13-27 Kasuga, Bunkyo-ku Tokyo 112-8551, Japan
Keywords:
Image Processing, Retina, Fractional Calculus.
Abstract:
The first part of a cone’s signal transduction is investigated from an image processing perspective in order to
find out what differentiates (human) vision from computer vision. We found that the activity of cone opsins—
visual pigments that are activated by the impact of a photon—can be described as an approximation of a
fractional integrator of order 0.1–0.2 on frequencies between 1–30 Hz. We explore how this affects the output
signal and provide examples of how this can be used for noise reduction and image processing. We also
present a simplified model since these processes require excessive computational power for computer vision
modeling.
1 INTRODUCTION
Human vision is so reliable that it can override all
other senses when conflicting inputs are presented;
however, the same cannot be said of computer vision.
A number of papers draw inspiration from human vi-
sion image processing, most of which model the net-
work of retinal cells or part of the brain (Bratkova
et al., 2009; Gould et al., 2007; Eeckman, 1989;
Means, 1992; Yang et al., 2013).
The goal of this paper is to explore signal forma-
tion within the retina’s cones after the impact of a
photon to see how it affects the resulting signal and
any subsequent image processing. Because the set
of equations that describe this process require exces-
sive computational power, it is necessary to determine
what characterizes these equations to simplify them
successfully.
According to our results, the equations that de-
scribe the activity of cone opsins behave as a frac-
tional integrator I
0.2
on frequencies between 1–30 Hz.
This means that it acts as a first order system—a low
pass filter—above 30 Hz, and signals above 1 Hz are
attenuated as well to a lesser extent.
2 MODELING
2.1 Signal Transduction of the Cones
Signal transduction is the formation of a cell’s signal
in response to an external stimulus. A complete re-
view of this process is beyond the scope of this paper,
so only the relevant parts will be reviewed: the acti-
vation and inactivation of cone opsins.
Because it is difficult to directly measure concen-
trations within a cell, there are controversial parts
of this process such as inactivation rates (Gross and
Burns, 2010; Korenbrot, 2012b; Invergo et al., 2013a;
Zhang, 1997) and the number of phosphorylation sites
(Korenbrot, 2012a; Qu and Vondriska, 2009). There-
fore, one of the goals of this study is to determine
what kind of behavior is expected from these kinds of
equations and processes.
2.1.1 Activation and Inactivation
The impact of a photon in a cone activates a visual
pigment (VP). When active, VPs activate their cor-
responding G-proteins (“transducins”). The inactiva-
tion of an active VP (VP*) consists of several steps.
First, multiple phosphorylations by a G-protein ki-
nase (GPK) occur, where each phosphorylation halves
its ability to activate nearby transducins (Adamus
et al., 1993; De Palo et al., 2013; Invergo et al., 2013b;
Reiter and Lefkowitz, 2006). Next, phosphorylated
VP*s are inactivated by arrestin, the rate of which de-
pends on how many times the VP* has been phospho-
rylated.
Inactivate VPs (VP
0
s) still have activity between
5% and 50% compared to the previous stage (Ascano
and Robinson, 2006; Sinha et al., 2014). Finally, the
binding of arrestin targets VP
0
s for internalization,
i.e., recycling it back to its default state. During this
481
Martinecz A. and Niitsuma M..
Modeling the G-Protein Signaling of the Retina with Fractional Calculus.
DOI: 10.5220/0005515304810488
In Proceedings of the 12th International Conference on Informatics in Control, Automation and Robotics (ICINCO-2015), pages 481-488
ISBN: 978-989-758-122-9
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)
process, its activity (as a “free-opsin”) is reduced to
nearly zero, approximately 2.5·10
5
–10
6
times weaker
than the activity of an unphosphorylated VP* (Corn-
wall and Fain, 1994; Lamb and Pugh, 2004; Fan et al.,
2005). Based on the differences in spontaneous activ-
ities in cones and rods, this should be a higher value
(Yau and Hardie, 2009).
An active transducin form a complex with an inac-
tive phosphodiestarese (PDE) making it active as long
as it is bound. Active transducins are inactivated by
the RGS9 complex, which makes it dissociate from
the PDE, thereby halting its activity.
2.1.2 Adaptation
Adaptation is mostly controlled by processes down-
stream; for example, by the concentration of Ca++
within the cell (Invergo et al., 2013b; Arshavsky and
Burns, 2012). In this paper, we include the adapta-
tion that affects the maximum rate of VP* phospho-
rylation (Korenbrot, 2012a). With higher inputs, the
phosphorylation rate is decreased making the output
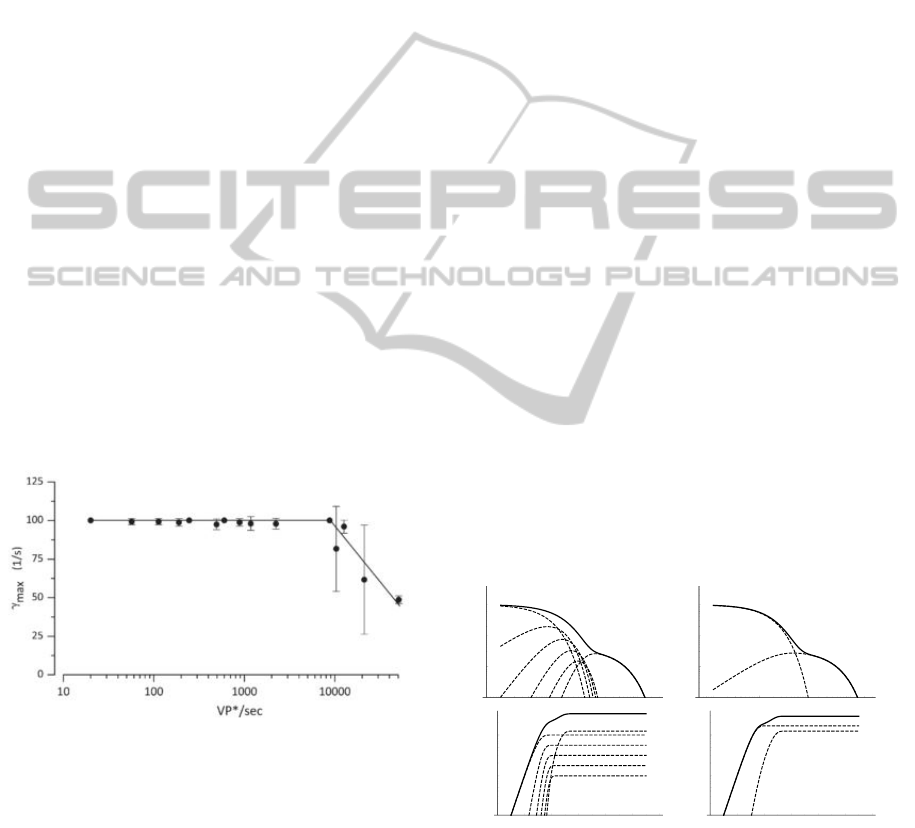
even higher as shown in Fig. 1.
The values of the adaptation were determined sep-
arately for an impulse response and for a step re-
sponse in (Korenbrot, 2012a). In our model, the adap-
tation is dependent on the number of active VP*s
within the simulated cell, this adequately approxi-
mates both the impulse and step response adaptation
of the model described in (Korenbrot, 2012a).
Figure 1: Adaptation of γ, depending on the number of
VP*/sec on the input (step response version) (Korenbrot,
2012a).
2.1.3 Equations
We have opted to use the mathematical model of (Ko-
renbrot, 2012b; Korenbrot, 2012a). Because of the
range of possible parameters for VP
0
and free opsins
in the literature, we have chosen not to extend the
model to include them.
Let γ be the rate of phosphorylation; it’s value
ranges between 50 and 100 s
−1
depending on adapta-
tion. Furthermore, n
PDE
is the number of active PDEs,
the same as the number of active transducins in this
model.
˙x
0
= input(t) − γ · x
0
(1)
˙x
1
= γ · x
0
− (γ · 0.9 + 0.5)x
1
(2)
˙x
2
= γ · 0.9x
1
− (γ · 0.9
2
+ 2 · 0.5)x
2
(3)
.
.
.
˙x
6
= (γ · 0.9
5
+ i · 0.5)x
5
− 5 · 0.5x
6
. (4)
The output (number of active PDEs) is:
˙n
PDE
= 230(
∑
i
2
−i
x
i
) − 12.5 · n
PDE
. (5)
Arrestin binding is negligible with the exception
of the state after the last phosphorylation since the
phosphorylation is 20-fold faster. In steady state, each
level of phosphorylation has 1.11 times more VP*s
than the previous level, and since each successive
level’s activity is halved, they can be replaced with
just one level with the activity of the first level and
pole of the last phosphorylation. A comparison is
shown in Fig. 2. The simplified model for six phos-
phorylation sites is:
˙x
0
= input(t) − γ · 0.9
6
· x
0
(6)
˙x
1
= γ · 0.9
6
· x
0
− 3x
1
. (7)
The output is:
˙n
PDE
= 230(x
0
+ 2
−5
x
1
) − 12.5n
PDE
. (8)
10
-3
10
-2
0.1 1
t (sec)
10
-2
0.1
1
10
-3
10
-2
0.1 1
t (sec)
10
-2
0.1
1
10
-5
10
-3
0.1 10 1000 10
5
10
7
t (sec)
5. × 10
-4
1. × 10
-3
5. × 10
-3
1. × 10
-2
10
-5
10
-3
0.1 10 1000 10
5
10
7
t (sec)
5. × 10
-4
1. × 10
-3
5. × 10
-3
1. × 10
-2
Figure 2: Impulse (top) and step (bottom) responses of the
original (left) and simplified (right) models on a log–log
scale. Solid curves represent the output, and dashed lines
represent each feedback loop’s contribution.
2.1.4 Range of Values
The time course from VP activation to VP* is negli-
gible, specifically, it is 1 ms (Korenbrot, 2012b).
ICINCO2015-12thInternationalConferenceonInformaticsinControl,AutomationandRobotics
482

The lowest detectable values in cones can be pro-
duced by 4–10 unphosphorylated VP*s.
There are 10
8
VPs in a rod, so we assume that
cones have a similar number of VPs (Lamb and Pugh,
2004). A flash of light activates 1–10
6
VP/sec (Ko-
renbrot, 2012b).
This model does not address sensitivity loss due to
the decrease of available VPs since the internalization
process takes a considerable amount of time.
2.2 Fractional Calculus
Since the structure of this process is self-similar, we
hypothesize that these equations describe a fractional
integral (Charef et al., 1992; Clerc et al., 1984; Dalir
and Bashour, 2010). In fractional calculus, instead
of integer order integrals and derivatives (for exam-
ple, I
1
, I
5
, D
2
, D
−13
), any order can be defined (for
example, I
0.32
, I
π
, D
4+2i
). In this paper, we restrict
ourselves to the fractional integrals of a real order be-
tween 0 and 1.
An interpretation of a fractional integral is the
distortion of the time scale during integration: time
“slows down” as it approaches the present, therefore,
giving it more weight (Podlubny, 2002). The opposite
interpretation is that past values are being gradually
“forgotten”, and therefore, having less weight.
The Riemann-Liouville definition of the fractional
integral is
I
α
f (x) =
1
Γ(α)
Z
b
a
f (t)(b −t)
α−1
dt. (9)
Using convolution, this can be converted to a more
convenient form for those familiar with control the-
ory:
I
α
f (x) =
1
Γ(α)
f (t) ⊗t
α−1
. (10)
If 0 < α < 1, the impulse response of a fractional
integrator is (1/t)
c
. Compared to a feedback loop’s
exponential e
−t
on a logarithmic scale, the slope of
the function does not change over the logarithm of
time; therefore, the time it takes for the signal to
be undetectable greatly depends on the input signal’s
strength (see Fig. 3).
2.2.1 Approximation
Since as an operation the fractional integral is linear,
it can be approximated by approximating its impulse
response. The gamma function from a signal process-
ing standpoint is just a gain for the whole operation
therefore it can be neglected. In order to show that the
equations that describe the activity of cone opsins act
0.1 1 10 100
t(sec)
10
-4
10
-3
10
-2
0.1
1
10
Figure 3: Exponential decay (g(t) = e
−t
, solid curve) and
power-law decay ( f (t) = 1/t, dashed line) on a log–log plot.
as a fractional integral, it suffices to show that a frac-
tional integral’s impulse response is approximated by
the system.
In log–log scale, its impulse response is linear;
therefore, it can be approximated by a piecewise con-
stant function. For example, signals that occur
• 0.1–1 s ago have an average weight ∼ 0.3
α−1
,
• 1–10 s ago have an average weight ∼ 3
α−1
,
• 10–100 s ago have an average weight ∼ 30
α−1
,
and so on, where α is the order of the fractional
integral (see Fig. 4).
0.1 1 10 100 1000 10
4
t(sec)
0.05
0.10
0.50
1
5
Figure 4: Piecewise approximation (solid lines) of f (t) =
t
−0.5
(dashed) on a log–log plot.
Connected feedback loops form similar groups to
the piecewise function mentioned above. This also re-
duces the necessary memory for the operation to only
a few variables, even on extended timescales such as
minutes.
With differential equations:
˙x
0
= input(t) − ax
0
(11)
˙x
1
= ax
0
− bx
1
(12)
˙x
2
= bx
1
− cx
2
(13)
.
.
..
ModelingtheG-ProteinSignalingoftheRetinawithFractionalCalculus
483

The output is the weighted sum of x
i
:
y =
∑
i
(c
i
x
i
). (14)
Since we want to exploit the self-similar nature of
this model on the log–log plot, the poles of the feed-
back loops have to decrease logarithmically (100, 10,
1, ..., etc.), or at least it has to be self-affine (100, 8,
1.5, ..., etc.).
A feedback loop’s settling time is 3–4 times
its time constant depending on the definition used:
within 5% or 2% of the final value. We assume the
ith loop receives an impulse input at t = 0 and has a
time constant of τ
i
= 1. If the next loop’s time con-
stant τ
i+1
τ
i
, the maximum of the next loop will be
between approximately 2τ
i
and 3τ
i
.
If τ
i+1
= 10τ
i
, to find the lower bound of x
i+1
’s
maximum, the following approximation is used: at
t = 0, it receives all the inputs it would receive until
its maximum: t =2τ
i
...3τ
i
. While this ignores the fact
that x
i+1
is gradually filled, it is a good estimate for
finding the time it reaches its maximum.
x
i+1
(0) = 1 − x
i
(2...3τ
i
). (15)
The maximum is at t = 2.5τ
i
, where the second
loop is at least 71.5% full (maximum is at 2.5τ
i
):
x
i+1
(2.5τ
i
) ≈ (1 − e
−2.5
) · e
−2.5/10
= 0.7148. (16)
While this may be below the desired value, the
influence of the previous loop will still have an effect
on the output as shown in Fig. 5. After 3τ
i
, the i+1st
loop receives little to no input from the previous loop
and decays exponentially in the same manner as the
ith loop. Since the system is self-affine, the i+2nd
loop can be calculated the same way.
The weight of each loop depends on the maximum
of each loop’s impulse response. To approximate a
fractional integral, the maximums have to form a line
on the log–log plot.
0.1 10 1000 10
5
t(sec)
0.2
0.5
1
2
5
Figure 5: Approximation without different weights on the
log–log plot. Gray lines represent the contribution of each
loop, and the black curve is the output.
log
c
i
c
i−1
= (α − 1)log
t
max,i
t
max,i−1
, (17)
where α is the order of integration, and t
max,i
is the
time to each loop’s peak.
Assuming that the next loop’s maximum is x · τ
i
(some multiple of τ):
t
max,i
≈ x
i
∑
k=0
τ
k
. (18)
For even logarithmic spacing (τ
i
= τ
i
0
), the maxi-
mums form a geometric series:
t
max,i
≈ x
i
∑
k=0
τ
k
= x
1 − τ
(i+1)
0
1 − τ
0
. (19)
Hence, (17) can be rewritten as
log
c
i
c
i−1
= (α − 1)log
1 − τ
(i+1)
0
1 − τ
i
0
. (20)
In other words, the approximation is independent
of x (the relative place of the maximums).
If τ
0
≥ 5 and τ
i
= τ
i
0
, the displacement of the max-
imums can be neglected as well:
log
c
i
c
i−1
≈ (α − 1). (21)
Overall, if the poles are sufficiently far and spaced
logarithmically, then the ith loop’s pole and gain will
dominate the given impulse response on its timescale.
10
-3
1 1000 10
6
t(sec)
10
-5
10
-3
10
-1
Figure 6: Approximation of f (t) = t
−0.5
with feedback
loops on the log–log plot. Gray lines represent the con-
tribution of each loop, and the black curve is the output.
3 RESULTS
3.1 Approximation Example
Consider the approximation of I
α
where the poles are
p
i
= 10
i
and five feedback loops are used (the bare
minimum is two loops). Strictly speaking Γ(α) · I
α
,
ICINCO2015-12thInternationalConferenceonInformaticsinControl,AutomationandRobotics
484
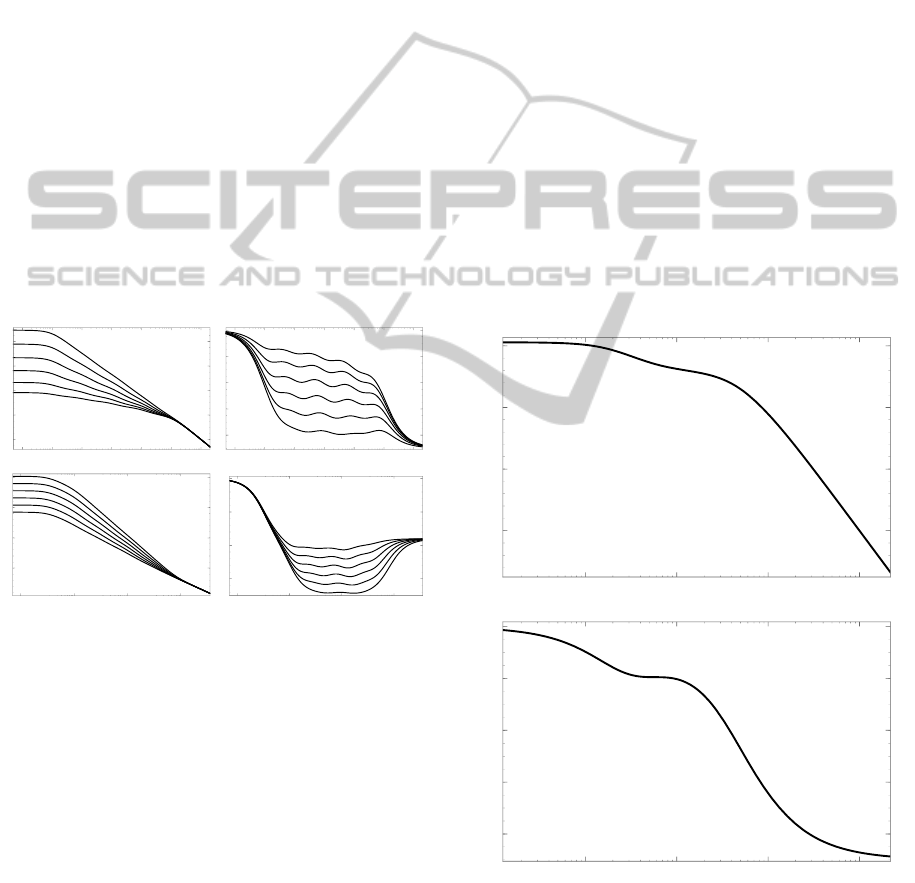
since for signal processing the gamma function was
neglected. The state space representation is:
A =
−1 0 0 0 0
1 −0.1 0 0 0
0 0.1 −0.01 0 0
0 0 0.01 −0.001 0
0 0 0 0.001 −0.0001
B =
1 0 0 0 0
T
C =
1 0.1
α−1
0.1
2(α−1)
0.1
3(α−1)
0.1
4(α−1)
.
The Bode plots of fractional integrals have a slope
of −20α dB/dec and a constant phase of -90α deg
(Podlubny et al., 2002; Sierociuk et al., 2013). Both
Bode plots and impulse responses fully define a linear
time invariant system.
In case of the approximation as α approaches zero
(the identity operation), the approximation gets less
and less reliable. The algorithm works for α > 1 as
well; however, outside the chosen frequency band, the
system behaves as a first-order system not as dαe as
shown in Fig. 7.
10
-5
10
-4
0.001 0.01 0.1 1 10
-20
0
20
40
60
Magnitude[dB/dec]
10
-5
10
-4
0.001 0.01 0.1 1 10
-80
-60
-40
-20
0
Phase [deg]
10
-5
0.001 0.1 10
0
50
100
150
Magnitude[dB/dec]
10
-5
0.001 0.1 10
-150
-100
-50
0
Phase [deg]
Figure 7: Bode plots of the approximations of I
0.15
, I
0.3
,
I
0.45
, I
0.6
, I
0.75
, I
0.9
(top) and I
1.15
, I
1.3
, I
1.45
, I
1.6
, I
1.75
,
I
1.9
(bottom).
Note that imperfect data transfer has a similar ef-
fect to having reduced weights since it will reduce the
gain of the subsequent loops.
3.2 Signaling of Cone Opsins
We have shown that the signaling of cone opsins in
Eq. (6) and (7) can approximate a fractional integral;
in particular, they describe an approximation with an
order of 0.2 between 1–30 Hz as shown in Fig. 8.
An extended model involving VP
0
s and free
opsins also fits the approximation on a broader range
between 0.1–30 Hz with an order of 0.1 (depending
on the values used). However, it is difficult to prove
which values are appropriate without further compar-
ison to biological data; therefore, we have chosen not
to include it in our demonstrations for now.
This kind of power-law behavior in cells is not
unheard of; for example, it is theorized that there
is a power-law component of the auditory nerves’
adaptation (Zilany and Carney, 2010). The activ-
ity of vestibulo-oculomotor neurons is described with
fractional calculus in (Anastasio, 1994; Thorson and
Biederman-Thorson, 1974).
To see how power-law behavior affects video
input, simulations were conducted using Wolfram-
Mathematica 10 and the nVidia CUDA framework.
The simplified cone equations with adaptation in-
cluded were simulated for each cell. Based on step
responses, the output image was determined to be
the sum of the following two images with different
weights depending on adaptation:
• imageA: an image that is almost the same as one
frame from a 30 Hz camera,
• imageB: an image that is the sum of the past ap-
proximately 30 frames.
1 10 100 1000
-60
-50
-40
-30
Magnitude[dB/dec]
1 10 100 1000
-80
-60
-40
-20
0
Phase [deg]
Figure 8: Bode plots of the simplified model (γ = 100 s
−1
).
Note the constant phase around 18 deg.
ModelingtheG-ProteinSignalingoftheRetinawithFractionalCalculus
485

3.3 Image Processing
As input, a game and movie trailer were used: the
“Harry Potter: Half-Blood Prince” trailer and the
“World of Warcraft: Wrath of the Litch King” trailer.
Both have eye tracking data available at (Mital et al.,
2011) for later parts of research.
It should be kept in mind during the evaluation
that eye movements have unique properties; specifi-
cally, they are characterized as ”ballistic”: fast move-
ments followed by short stops. As a natural mech-
anism, the eyes tend to focus on certain points of
moving objects, which means that the scene in focus
should only change by 2–5 Hz. This has not been
modeled at this stage of our research.
3.4 Noise Reduction
Assuming a Gaussian distribution of temporal noise,
unlike imageA, imageB contains little to no noise. For
example, refer to the snowflakes in Fig. 9. A feedback
loop as a low-pass filter for noise reduction is inef-
ficient because quickly moving parts are blurred and
often “invisible”, however in the case of the fractional
integral they can still be detected because of imageA
as shown in Fig. 10.
The adaptive process changes the ratio of imageA
and imageB by increasing the lifetime of VP
0
s for
stronger inputs. This means that brighter scenes (and
pixels) have less noise reduction. The effects of the
non-linearity produced by this adaptation are difficult
to detect. Similarly to a camera, darker scenes would
contain more noise otherwise. The bright areas be-
come 50% brighter; however, after scaling back the
values between 0–255 for display and print purposes,
the introduced non-linearity becomes invisible, see
Fig. 10.
3.5 Partially Obstructed Images
As a special case, in the Harry Potter trailer, there is
a scene where he runs in tall grass. Since the cam-
era follows him, it is similar to simulated eye move-
ment (however, vertical movement is not stabilized).
On multiple frames, his face is partially obstructed by
leaves, so an object or face recognition algorithm may
be difficult to apply. However, because of imageB,
there is a faint outline of his face that remains from
previous frames as can be seen in Fig. 11. While this
is is an unusual problem, we believe that it is repre-
sentative of the flexibility of human vision.
Figure 9: Scene from the World of Warcraft trailer (top).
After processing (with adaptation), the same snowfall is
barely visible (bottom).
Figure 10: Scene from the World of Warcraft trailer (top).
With adaptation, compared to the original, there is little to
no difference in the brightness values (bottom).
4 FUTURE DIRECTIONS
There are two directions this research will take: eye
movements and investigating the full model of cones.
4.1 Patterns
In (Sligte et al., 2008), it is hypothesized that there
ICINCO2015-12thInternationalConferenceonInformaticsinControl,AutomationandRobotics
486

Figure 11: Three frames from the original Harry Potter
trailer (left column). After processing—because of the
afterimage—parts of his face are no longer obstructed by
leaves (right column).
are three visual short-term memory storages. Of the
three, short-term iconic memory is most relevant to
this paper since it stores visual information in posi-
tive afterimages for brief periods of time. There is
a distinction between rods and cones; rods provide
a stronger afterimage because of their slow response
(there is basically only imageB). This type of mem-
ory has been tested by them using different masking
images to erase the afterimage provided by the cones,
leaving the afterimage provided by the rods and vice-
versa. Short-term iconic memory is weaker in cones
although still present. This is consistent with our find-
ings, as cones still provide a weak but noticeable af-
terimage. Eye movements will be used in later parts
of our research to assess their basic function and po-
tential for image processing.
For finding breaks in a pattern, it is the easiest to
subtract two subsequent frame; however in biological
systems this operation is a bit more difficult. Our hy-
pothesis is that since eye movements cannot instanta-
neously compare two images by subtraction, buffer-
ing the previous frame is not enough since it only
contains data about eye movements. Moreover, it is
not known when and what information will be useful
in the future, so storing a second of data in imageB
makes the most sense. For this operation a substan-
tial overshoot is needed in the next processing steps.
Bipolar cells for example are the next row of cells af-
ter cones and rods and get the cones’ output as input.
Their impulse responses substantially overshoot, so
any difference between imageA and imageB will be
highlighted (Ichinose et al., 2014; Wassle, 2004).
4.2 Curves, Parallel Lines
When focusing on lines (edges), the eyes will focus
on the points of the line instead of focusing on an
empty space next to the line. When changing focus
from one point to another on the line, an overshoot
in the subsequent processing steps will highlight the
differences between imageA and imageB. Therefore
it will be very apparent if the line is not completely
straight or if nearby edges/lines are not parallel.
5 CONCLUSIONS
There is a difference between the data processing of
the retina and traditional computer-based image pro-
cessing; the retina has video processing, and individ-
ual cones and rods process input as a function of time.
Moreover, the network of cones affect each other,
and their output depend on all the values of the past
minute (as well as the horizontal, amacrine, bipolar,
and ganglion cells downstream). We have shown how
this affects video input and how it might be utilized by
our eyes. We also discussed why eye movements are
important and how they affect this kind of processing.
Based on our findings, we believe that processing a
video as a whole instead of frame-by-frame has great
potential.
REFERENCES
Adamus, G., Arendt, A., Hargrave, P. A., Heyduk, T., and
Palczewski, K. (1993). The kinetics of multiphospho-
rylation of rhodopsin. Archives of biochemistry and
biophysics, 304(2):443–7.
Anastasio, T. J. (1994). The fractional-order dynamics of
brainstem vestibulo-oculomotor neurons. Biological
cybernetics, 72(1):69–79.
Arshavsky, V. Y. and Burns, M. E. (2012). Photoreceptor
signaling: supporting vision across a wide range of
light intensities. The Journal of biological chemistry,
287(3):1620–6.
Ascano, M. and Robinson, P. R. (2006). Differential phos-
phorylation of the rhodopsin cytoplasmic tail mediates
the binding of arrestin and its splice variant, p44. Bio-
chemistry, 45:2398–2407.
Bratkova, M., Boulos, S., and Shirley, P. (2009). oRGB: a
practical opponent color space for computer graphics.
IEEE computer graphics and applications, 29(1):42–
55.
Charef, A., Sun, H., Tsao, Y., and Onaral, B. (1992).
Fractal system as represented by singularity func-
tion. IEEE Transactions on Automatic Control,
37(9):1465–1470.
ModelingtheG-ProteinSignalingoftheRetinawithFractionalCalculus
487

Clerc, J., Tremblay, A.-M., Albinet, G., and Mitescu, C.
(1984). a.c. response of fractal networks. Journal de
Physique Lettres, 45(19):913–924.
Cornwall, M. C. and Fain, G. L. (1994). Bleached pigment
activates transduction in isolated rods of the salaman-
der retina. The Journal of physiology, 480 ( Pt 2:261–
79.
Dalir, M. and Bashour, M. (2010). Applications of
fractional calculus. Applied Mathematical Sciences,
4(21):1021–1032.
De Palo, G., Facchetti, G., Mazzolini, M., Menini, A.,
Torre, V., and Altafini, C. (2013). Common dynamical
features of sensory adaptation in photoreceptors and
olfactory sensory neurons. Scientific reports, 3:1251.
Eeckman, F. (1989). A retina-like model for motion de-
tection. In International Joint Conference on Neural
Networks, pages 247–249 vol.2. IEEE.
Fan, J., Woodruff, M. L., Cilluffo, M. C., Crouch, R. K., and
Fain, G. L. (2005). Opsin activation of transduction in
the rods of dark-reared Rpe65 knockout mice. The
Journal of physiology, 568(Pt 1):83–95.
Gould, S., Arfvidsson, J., Kaehler, A., Sapp, B., Messner,
M., Bradski, G., Baumstarck, P., Chung, S., and Ng,
A. Y. (2007). Peripheral-foveal vision for real-time
object recognition and tracking in video. In Inter-
national Joint Conference on Artificial Intelligence,
pages 2115–2121. Morgan Kaufmann Publishers Inc.
Gross, O. P. and Burns, M. E. (2010). Control of
rhodopsin’s active lifetime by arrestin-1 expression
in mammalian rods. The Journal of neuroscience :
the official journal of the Society for Neuroscience,
30(9):3450–7.
Ichinose, T., Fyk-Kolodziej, B., and Cohn, J. (2014). Roles
of ON Cone Bipolar Cell Subtypes in Temporal Cod-
ing in the Mouse Retina. Journal of Neuroscience,
34(26):8761–8771.
Invergo, B. M., Montanucci, L., Koch, K.-W., Bertranpetit,
J., and Dell’orco, D. (2013a). Exploring the rate-
limiting steps in visual phototransduction recovery by
bottom-up kinetic modeling. Cell communication and
signaling : CCS, 11(1):36.
Invergo, B. M., Montanucci, L., Laayouni, H., and Bertran-
petit, J. (2013b). A system-level, molecular evolution-
ary analysis of mammalian phototransduction. BMC
evolutionary biology, 13:52.
Korenbrot, J. I. (2012a). Speed, adaptation, and stability
of the response to light in cone photoreceptors: the
functional role of Ca-dependent modulation of ligand
sensitivity in cGMP-gated ion channels. The Journal
of general physiology, 139(1):31–56.
Korenbrot, J. I. (2012b). Speed, sensitivity, and stability
of the light response in rod and cone photoreceptors:
Facts and models. Progress in retinal and eye re-
search, 31(5):442–466.
Lamb, T. D. and Pugh, E. N. (2004). Dark adaptation and
the retinoid cycle of vision. Progress in retinal and
eye research, 23(3):307–80.
Means, R. (1992). Neural Network Retinal Model Real
Time Implementation.
Mital, P. K., Smith, T. J., Hill, R., and Henderson, J. M.
(2011). Clustering of gaze during dynamic scene
viewing is predicted by motion. Cognitive Compu-
tation, 3(1):5–24.
Podlubny, I. (2002). Geometric and Physical Interpreta-
tion of Fractional Integration and Fractional Differ-
entiation. Fractional Calculus and Applied Analysis,
5(4):367–386.
Podlubny, I., Petra
ˇ
s, I., and Vinagre, B. (2002). Analogue
realizations of fractional-order controllers. Nonlinear
dynamics, pages 281–296.
Qu, Z. and Vondriska, T. M. (2009). The effects of cas-
cade length, kinetics and feedback loops on biological
signal transduction dynamics in a simplified cascade
model. Physical biology, 6(1):016007.
Reiter, E. and Lefkowitz, R. J. (2006). GRKs and beta-
arrestins: roles in receptor silencing, trafficking and
signaling. Trends in endocrinology and metabolism:
TEM, 17(4):159–65.
Sierociuk, D., Podlubny, I., and Petras, I. (2013). Experi-
mental Evidence of Variable-Order Behavior of Lad-
ders and Nested Ladders. IEEE Transactions on Con-
trol Systems Technology, 21(2):459–466.
Sinha, A., Jones Brunette, A. M., Fay, J. F., Schafer, C. T.,
and Farrens, D. L. (2014). Rhodopsin TM6 can in-
teract with two separate and distinct sites on arrestin:
Evidence for structural plasticity and multiple docking
modes in arrestin-rhodopsin binding. Biochemistry,
53:3294–3307.
Sligte, I. G., Scholte, H. S., and Lamme, V. a. F. (2008). Are
there multiple visual short-term memory stores? PloS
one, 3(2):e1699.
Thorson, J. and Biederman-Thorson, M. (1974). Distributed
relaxation processes in sensory adaptation. Science
(New York, N.Y.), 183(4121):161–72.
Wassle, H. (2004). Parallel processing in the mam-
malian retina. Nature reviews. Neuroscience,
5(October):747–757.
Yang, K., Gao, S., Li, C., and Li, Y. (2013). Effi-
cient Color Boundary Detection with Color-Opponent
Mechanisms. 2013 IEEE Conference on Computer Vi-
sion and Pattern Recognition, pages 2810–2817.
Yau, K. and Hardie, R. (2009). Phototransduction motifs
and variations. Cell, 139(2):246–264.
Zhang, L. (1997). Rhodopsin Phosphorylation Sites and
Their Role in Arrestin Binding. Journal of Biological
Chemistry, 272(23):14762–14768.
Zilany, M. S. a. and Carney, L. H. (2010). Power-law dy-
namics in an auditory-nerve model can account for
neural adaptation to sound-level statistics. The Jour-
nal of neuroscience, 30(31):10380–90.
ICINCO2015-12thInternationalConferenceonInformaticsinControl,AutomationandRobotics
488
