
Influence of 532 and 355 nm Nanosecond Laser Pulses on
Photodestruction of Silver Nanoparticles in Photo-thermo-refractive
Glasses
Alexander Ignatiev, Dmitry Ignatiev, Dmitry Klyukin, Nikolay Nikonorov, Rustam Nuryev and
Alexander Sidorov
ITMO University, Kronverksky 49, St. Petersburg, Russia
Keywords: Nanosecond Laser, Silver Nanoparticle, Glass, Photodestruction.
Abstract: In this research we investigate an influence of wavelength of nanosecond laser radiation on the process of
silver nanoparticles photodestruction in photo-thermo-refractive glass. Second and third harmonic of
nanosecond YAG:Nd laser have been applied to irradiate photo-thermo-refractive silicate glasses with silver
nanoparticles and different halogenides (F, Cl, Br) as dopants. Optical spectroscopy and X-ray diffraction
analysis have revealed a presence of core/shell nanoparticles Ag/AgBr and Ag/Na
0.8
Ag
0.2
Cl. Irradiation of
samples by third harmonic at 355 nm wavelength causes a red shift of surface plasmon resonance band (35
nm) whereas nanosecond laser radiation at 532 nm does not cause significant shift of the surface plasmon
resonance band. Such a difference is caused by mechanisms involved in the photodestruction process.
1 INTRODUCTION
Phototermorefractive (PTR) glasses are perspective
material for recording of highly efficient volume
phase holograms operating in red visible and near IR
spectral range (700-3000 nm) (Dubrovin et al., 2014;
Ignatiev et al., 2015). The PTR-glasses allow to
precipitate silver nanoparticles (SNPs) possessing
high absorption coefficient in visible spectral range
(λ
max
=414-490 nm) and nanosize crystalline phase of
NaF in local area of the glass host by photo-thermo-
induced crystallization (Nikonorov et al., 2010).
Unfortunately, this absorption band of SNPs restrict
to the using the holograms in short visible range.
There are several investigation devoted to the
photodestruction of silver nanoparticles in glass
matrix. An influence of femtosecond laser radiation
on SNPs in glass matrix after ion-exchange was
widely investigated (Podlipensky et al., 2004;
Stalmashonak et al., 2007). However, the
mechanisms of photodestruction of relatively big
nanoparticles larger than 20 nm differ from the small
ones and require particular attention. The influence
of laser irradiation with wavelength 532 nm was
well described for silver-containing silicate glass.
Nevertheless, it is still very important to use a laser
radiation with an appropriate wavelength to reduce
the possible damage to the hologram efficiency in
such glasses. In the present work we demonstrated a
possibility of reduction of the absorption band by
bleaching technology with the use of pulse (9 ns)
laser radiation with two wavelengths at 355 and 532
nm. The features of SNPs with different shells and
surrounding in glass matrix are considered using X-
ray diffraction analysis and optical spectroscopy.
2 EXPERIMENTAL
In our studies we have used PTR glass of sodium-
alumina-silicate system, Na
2
О – Al
2
O
3
– ZnO – SiO
2
– NaF –NaCl(Br), activated by CeO
2
(0.007 mol%),
Sb
2
O
3
(0.02 mol%), and Ag
2
O(0.007 mol%).
The glasses were synthesized in fused silica
crucibles at 1500 °С in the environment air. Stirring
with a Pt thimble was used to homogenize the liquid.
After melting, the glasses were cooled down to 500
°С , then annealed at glass transition temperature (T
g
= 494 °С for Ag-Br and Ag-Cl samples, 473 °С for
Ag-Br-F sample) for 1h, and cooled down to room
temperature with a rate of 0.15K/min. The samples
were prepared as the polished plates with the
thickness 1 mm.
Ignatiev, A., Ignatiev, D., Klyukin, D., Nikonorov, N., Nuryev, R. and Sidorov, A.
Influence of 532 and 355 nm Nanosecond Laser Pulses on Photodestruction of Silver Nanoparticles in Photo-thermo-refractive Glasses.
DOI: 10.5220/0005669702410245
In Proceedings of the 4th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2016), pages 243-247
ISBN: 978-989-758-174-8
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
243
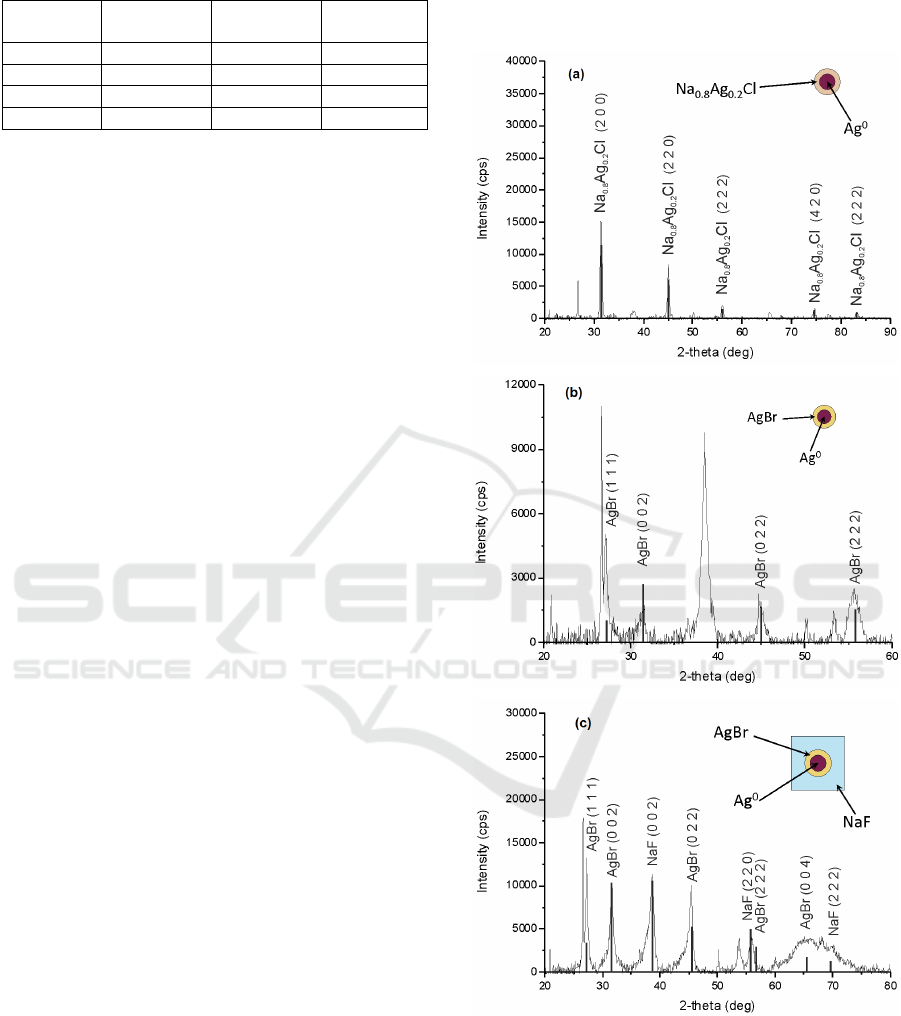
Table 1: Glass compositions.
Components Ag-Br,
(mol.%)
Ag-Cl,
(mol.%)
Ag-Br-F,
(mol.%)
Ag
2
O 0.006 0.006 0.006
Br 1.5 0 1.5
Cl 0 1.5 0
F 2 2 6
Samples were irradiated by high pressure mercury
lamp. Then a thermal treatment of samples was
carried out in programmable muffle furnaces
(Neibotherm) at 520 °С at 90 min. The irradiation of
the samples were carried out by pulsed (9 ns)
YAG:Nd
3+
laser TII LS-2131M (Lotis) with second
harmonic at 532 nm, pulse energy 70 mJ and third
harmonic at 355 nm, pulse energy 22 mJ at
frequency 10 Hz. The spectral measurements were
carried out by means of spectrophotometer Lambda
650 (Perkin-Elmer) in the range 200-800 nm with
the step of 1 nm. The X-ray diffraction spectra were
obtained on an Ultima IV diffractometer (Rigaku).
3 RESULTS AND DISCUSSIONS
After the mercury lamp irradiation and thermal
treatment, x-ray diffraction analysis of the samples
has been conducted. Fig.1a,b shows that Ag-Cl and
Ag-Br samples contain Na
0.8
Ag
0.2
Cl and AgBr
crystalline phase. It’s been suggested (Nikonorov et
al., 2010) that such a crystal can be formed at SNPs
as shell. The formation of NaF crystalline phase
occurs at relatively high concentration of F
-
ions (6
mol.%) and it happens only when glass composition
involves Cl or Br ions (Fig.1c). Therefore, it is
reasonable to suggest that depending on glass
composition SNPs have Na
0.8
Ag
0.2
Cl shell in Ag-Cl
sample, AgBr shell in Ag-Br sample and AgBr and
NaF shells in Ag-Br-F sample (inset on Fig.1a-c). A
significant decrease of glass refractive index after
UV irradiation and thermal treatment also proves the
formation of NaF surroundings (Ivanov et al., 2015).
Curves 1 and 2 at Fig.2 present optical density
spectra of investigated glasses before and after
mercury lamp irradiation and followed thermal
treatment. One can see that initially glasses were
colorless down to 350 nm. After the treatment an
absorption peak at 453 nm has appeared (Curve 1 at
Fig.1). This absorption is connected with surface
plasmon resonance (SPR) of SNPs in dielectric
matrix. Generally, SNPs are characterized by SPR
peak at 408-411 nm (Garcia, 2011), but in case of
Ag-Br and Ag-Br-F samples it is shifted to 453 nm
due to AgBr shell, which has larger value of
refractive index (Nikonorov et al., 2009). Maximum
of SPR peak for Ag-Cl samples is located at 419 nm.
Such a difference in SPR peak wavelength can be
Figure 1: (a) Ag-Cl sample X-ray diffraction data PTR
glasses containing 0.13 mol% Ag
2
O and 2.2 mol% NaCl
(b) Ag-Br sample X-ray diffraction data for PTR glasses
containing 0.06 mol% Ag2O and 1.5 mol% NaBr (c) Ag-
Br-F sample X-ray diffraction data for PTR glasses
containing 0.06 mol% Ag2O, 1.5 mol% NaBr and 6.0
mol% NaF.
PHOTOPTICS 2016 - 4th International Conference on Photonics, Optics and Laser Technology
244
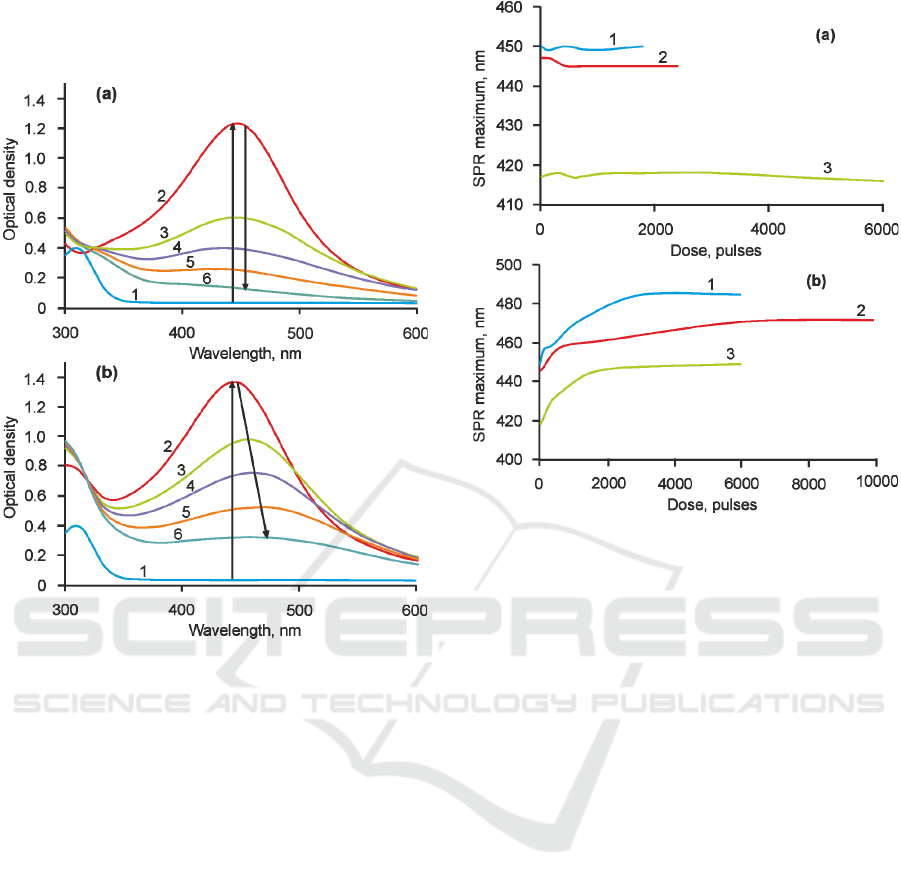
related with the width of halide shell or refractive
index of the shell (Nikonorov et al., 2009), but this
question won’t be considered in this research.
Figure 2: (a) Ag-Br-F after 532. 1- as-prepared glass, 2 –
before laser irradiation, 3 – 150 pulses, 4 – 300 pulses, 5 –
450 pulses, 6 – 1800 pulses. (b) Ag-Br-F after 355. 1 - as-
prepared glass, 2 – before laser irradiation, 3 – 600 pulses,
4 - 1800 pulses, 5 – 3000 pulses, 6 – 6000 pulses.
Following laser irradiation was conducted using
two harmonics of nanosecond laser at 532 and 355
nm with energy density 0.5 and 0.2 J/cm
2
respectively. Fig.2 shows the result of the action of
nanosecond laser pulses at 532 nm on Ag-Br-F
sample. One can see that the intensity of SPR peak
decreases with the increase of laser radiation dose,
whereas the SPR band position does not change. An
influence of nanosecond laser pulses at 355 nm
wavelength is differs significantly. Curves 3-8 also
shows the decrease of SNP band intensity
accompanied with its red shift on 30-35 nm. Also
there is a slight increase in absorption at 270-325 nm
spectral range, which can be associated with the
formation of glass network defects and a formation
of silver molecular clusters Ag
m
n+
(Klyukin et al.,
2014).
Figure 3: Influence of laser pulses with 532 nm (a) and
355 nm (b) wavelength on Ag-Br-F (1), Ag-Br (2) and
Ag-Cl (3) samples.
Fig.3 shows the influence of laser radiation dose
on the shift of SPR peak of each sample. One can
see that the similar behavior is observed for all the
glasses. Namely, the position of SPR peak doesn’t
alter significantly after laser pulses at 532 nm
(Fig.3a). Only Ag-Cl sample demonstrates slight
blue shift after 2000 pulses, which can be associated
with the decrease of refractive index around
nanoparticles or size of SNPs. However, the position
of SPR peak of SNPs alters dramatically during the
laser irradiation by pulses at 355 nm wavelength
(Fig.3b). Each sample demonstrates 10-15 nm red
shift after the first 500 pulses and it continues with
smaller rate until the glass is fully transparent. Table
2 contains the maximum SPR peak shift for each
sample. Clearly that only after the laser irradiation at
355 nm significant SPR peak shift occurs. It should
be noted that laser irradiation of the samples was
conducting until the full bleaching. Therefore, it is
possible to compare the rate of photobleaching of
the samples for particular pulse energy. For Ag-Cl
sample it took approximately 6000 pulses to bleach
it fully by laser radiation at 532 nm, whereas for Ag-
Br and Ag-Br-F the number of pulses doesn’t exceed
2500 pulses. So Ag-Br and Ag-Br-F glass samples
have an advantage to compare with Ag-Cl glass as
long as they can be photobleached by the
nanosecond laser at 532 nm almost 2 times faster.
Influence of 532 and 355 nm Nanosecond Laser Pulses on Photodestruction of Silver Nanoparticles in Photo-thermo-refractive Glasses
245

This result can be explained by closer position of
SPR peak to the laser wavelength at 532 nm, so the
probability of laser absorption much higher in case
of Br-containing samples with SPR peak near 450
nm. Nevertheless, in case of laser irradiation at 355
nm there is no similar dependence, where the
photobleaching process of Ag-Br sample takes
10000 pulses, whereas Ag-Br-F and Ag-Cl samples
become colorless even after 6000 pulses. It is also
reasonable to suggest that the photobleaching by
laser pulses at 532 nm wavelength took less pulses
because of higher energy density (0.5 J/cm
2
) to
compare with laser pulses at 355 nm (0.2 J/cm
2
).
Table 2: SPR band shift.
Laser
wavelength,
nm
Ag-Br, nm Ag-Cl, nm Ag-Br-F, nm
532 2 1 1
355 27 32 35
According to the theoretical research (Nikonorov et
al., 2009) the position of SPR peak depends on the
permittivity of the nanoparticle itself, its shell and
surrounding dielectric. It is assumed that SNP in the
investigated glasses are surrounded by high
refractive index shell (AgBr, Na
0.8
Ag
0.2
Cl), which
causes the initial SPR peak red-shifted position. The
following laser irradiation results in the
photodestruction of SNP, which can occur in
different ways (Hashimoto et al., 2012). It seems
that two main mechanisms of the photodestruction
take place: photothermal evaporation and Coulomb
explosion. Near-field ablation hardly involved in the
photodestruction of considered glasses as long as it
requires high intensity density, which can be
achieved rather by femtosecond laser pulses.
Therefore, it reasonable to explain the red shift of
SPR peak after laser irradiation at 355 nm by
photodestruction of SNP through the photothermal
evaporation of silver ions that come from the
nanoparticles core to the surroundings. That process
causes a local increase of refractive index of SNP
shell and following red-shift of SPR peak. This
process occurs gradually because the laser pulse
wavelength slightly overlap with SPR band.
Coulomb explosion mechanism is likely involved in
the photodestruction of SNPs by laser radiation at
532 nm. In this case SNPs are breaking at smaller
ones and as long as it occurs in condensed material
like glass with high value of viscosity the smaller
parts of SNPs can not overcome the glass matrix and
they stay near each other surrounded by shell with
the same refractive index. The reason of involving of
such mechanisms of photodestruction for particular
wavelengths is not quite understood yet.
4 CONCLUSIONS
In conclusion, we have demonstrated a possibility of
reduction of the absorption band by bleaching
technology with the use of pulse (9 ns) laser
radiation with two wavelengths at 355 and 532 nm.
X-ray diffraction analysis have revealed the
existence of AgBr, Na
0.8
Ag
0.2
Cl and NaF crystalline
phase in the investigated glasses after the mercury
lamp irradiation and following thermal treatment.
Such a crystalline phase is located around SNPs and
affects on the SPR band position. During the
irradiation the SNPs absorption band decreases
depending on the exposure dose. This process
accompanies with a red shift of SPR band (35 nm)
after the laser pulses at 355 nm, whereas there is no
significant shift of the absorption band after the laser
pulses at 532 nm. The photothermal evaporation is
responsible for the photodestruction of SNPs in case
of laser pulses at 355 nm, whereas the Coulomb
explosion can explain the results of the action by the
nanosecond laser pulses at 532 nm. The technology
allowed us to control the size of the silver
nanoparticeles in PTR glasses and record the phase
holograms in visible range.
ACKNOWLEDGEMENTS
This work was financially supported by Russian
Scientific Foundation (Agreement # 14-23-00136).
REFERENCES
Dubrovin, V.D., Ignatiev, A.I., Nikonorov, N. V., Sidorov,
A.I., Shakhverdov, T.A. & Agafonova, D.S., 2014.
Luminescence of silver molecular clusters in photo-
thermo-refractive glasses. Optical Materials, 36(4),
pp.753–759.
Garcia, M.A., 2011. Surface plasmons in metallic
nanoparticles: fundamentals and applications. Journal
of Physics D: Applied Physics, 44, p.283001.
Hashimoto, S., Werner, D. & Uwada, T., 2012. Studies on
the interaction of pulsed lasers with plasmonic gold
nanoparticles toward light manipulation, heat
management, and nanofabrication. Journal of
Photochemistry and Photobiology C: Photochemistry
Reviews, 13(1), pp.28–54.
Ignatiev, A.I., Ignatiev, D.A., Nikonorov, N. V. &
Sidorov, A.I., 2015. The influence of UV laser
PHOTOPTICS 2016 - 4th International Conference on Photonics, Optics and Laser Technology
246

radiation on the absorption and luminescence of
photothermorefractive glasses containing silver ions.
Optics and Spectroscopy, 119(2), pp.238–242.
Ivanov, S.A., Ignatiev, A.I. & Nikonorov, N. V., 2015.
Advances in photo-thermo-refractive glass
composition modifications. In M. Hrabovský, J. T.
Sheridan, & A. Fimia, eds. SPIE Optics +
Optoelectronics. International Society for Optics and
Photonics, p. 95080E.
Klyukin, D.A., Sidorov, A.I., Ignatiev, A.I. & Nikonorov,
N. V, 2014. Luminescence quenching and recovering
in photo-thermo-refractive silver-ion doped glasses.
Optical Materials, 38, pp.233–237.
Nikonorov, N. V., Sidorov, A.I., Tsekhomskiĭ, V.A. &
Lazareva, K.E., 2009. Effect of a dielectric shell of a
silver nanoparticle on the spectral position of the
plasmon resonance of the nanoparticle in
photochromic glass. Optics and Spectroscopy, 107(5),
pp.705–707.
Nikonorov, N.V., Sidorov, A.I. & Tsekhomskii, V.A.,
2010. Silver Nanoparticles. In D. P. Perez, ed. Silver
Nanoparticles. InTech, pp. 177–200.
Podlipensky, A. V., Grebenev, V., Seifert, G. & Graener,
H., 2004. Ionization and photomodification of Ag
nanoparticles in soda-lime glass by 150 fs laser
irradiation: A luminescence study. Journal of
Luminescence, 109, pp.135–142.
Stalmashonak, A., Seifert, G. & Graener, H., 2007.
Optical three-dimensional shape analysis of metallic
nanoparticles after laser-induced deformation. Optics
letters, 32(21), pp.3215–3217.
Influence of 532 and 355 nm Nanosecond Laser Pulses on Photodestruction of Silver Nanoparticles in Photo-thermo-refractive Glasses
247
