
A Topic-centric Approach to Detecting New Evidences for
Evidence-based Medical Guidelines
Qing Hu
1,2
, Zhisheng Huang
1
, Annette ten Teije
1
, Frank van Harmelen
1
, M. Scott Marshall
3
and Andre Dekker
3
1
Department of Computer Science, VU University Amsterdam, De Boelelaan 1081, Amsterdam, The Netherlands
2
College of Computer Science and Technology, Wuhan Univesity of Science and Technology, Wuhan, China
3
Department of Radiation Oncology (MAASTRO), Maastricht University Medical Centre, Maastricht, The Netherlands
Keywords:
Evidence-based Medical Guidelines, Medical Guideline Update, Semantic Distance, Context-awareness,
Topic-centric Approach.
Abstract:
Evidence-based Medical guidelines are developed based on the best available evidence in biomedical science
and clinical practice. Such evidence-based medical guidelines should be regularly updated, so that they can
optimally serve medical practice by using the latest evidence from medical research. The usual approach to
detect such new evidence is to use a set of terms from a guideline recommendation and to create queries for
a biomedical search engine such as PubMed, with a ranking over a selected subset of terms to search for
relevant new evidence. However, the terms that appear in a guideline recommendation do not always cover
all of the information we need for the search, because the contextual information (e.g. time and location, user
profile, topics) is usually missing in a guideline recommendation. Enhancing the search terms with contextual
information would improve the quality of the search results. In this paper, we propose a topic-centric approach
to detect new evidence for updating evidence-based medical guidelines as a context-aware method to improve
the search. Our experiments show that this topic centric approach can find the goal evidence for 12 guideline
statements out of 16 in our test set, compared with only 5 guideline statements that were found by using a
non-topic centric approach.
1 INTRODUCTION
Medical guidelines, or alternatively clinical guide-
lines, are conclusions or recommendations on the ap-
propriate treatment and care of people with specific
diseases and conditions, which are designed by med-
ical authorities and organizations. Evidence-based
medical guidelines are developed based on the best
available evidence in biomedical science and clini-
cal practice. Guideline recommendations in evidence-
based medical guidelines are annotated with their un-
derlying evidence and their evidence classes. Med-
ical guidelines have been proved to be valuable for
clinicians, nurses, and other healthcare professionals
(Woolf et al., 1999)
1
.
Ideally, a guideline should be updated immedi-
ately after new relevant evidence is published, so that
the updated guideline can serve medical practice us-
ing the latest medical research. However, because of
1
https://en.wikipedia.org/wiki/Medical guideline
the sheer volumes of medical publications, the up-
date of a guideline is often lagging behind medical
scientific publications. Not only are the number of
medical articles and the size of medical information
very large, but also they are updated very frequently.
For example, PubMed
2
alone contains more than 24
million citations for biomedical literature from MED-
LINE
3
. Thus, updating a guideline is laborious and
time-consuming.
In order to solve those disadvantages, some ap-
proaches have been proposed that use information re-
trieval or machine learning technology to find relevant
new evidence automatically. Reinders et al. (Rein-
ders et al., 2015) described a system to find relevant
new evidence for guideline updates. The approach
is based on MeSH terms and their TF-IDF weights,
which results in the following disadvantages: i)the
use of MeSH terms terms means that if a guideline
2
http://www.ncbi.nlm.nih.gov/pubmed
3
http://www.nlm.nih.gov/bsd /pmresources.html
282
Hu, Q., Huang, Z., Teije, A., Harmelen, F., Marshall, M. and Dekker, A.
A Topic-centric Approach to Detecting New Evidences for Evidence-based Medical Guidelines.
DOI: 10.5220/0005698902820289
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 5: HEALTHINF, pages 282-289
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

statement does not use any MeSH term, there is no
way to measure the relevance of a publication, ii)the
use of TF-IDF weights means that the system has to
gather all relevant sources, which is time-consuming,
iii)the number of returned relevant articles is some-
times too large (sometimes even a few million), so
that it is impossible for an expert to check if an evi-
dence is really useful for the guideline update. Irue-
taguena et al. (Iruetaguena et al, 2013) also developed
an approach to find new evidence. That method is
also based on gathering all relevant articles by search-
ing the PubMed website, and then uses the Rosenfeld-
Shiffman filtering algorithm to select the relevant ar-
ticles. The experiment of that approach shows the re-
call is excellent, but the precision is very low (10.000
articles contain only 7 goal articles) (Reinders et al.,
2015). In (Hu et al., 2015), we propose a method that
uses a semantic distance measure to automatically
find relevant new evidence for guideline updates. The
advantage of using semantic distance is that the rele-
vance measure can be achieved via the co-occurrence
of terms in a biomedical article, which can be eas-
ily obtained via a biomedical search engine such as
PubMed, instead of gathering a large corpus for the
analysis.
The existing approaches to detect relevant evi-
dence for guideline updates are using the terms ap-
pearing in a guideline statement. However, these
terms appearing in a guideline statement do not al-
ways cover all of the information we need for the
search, because the contextual information (e.g. time
and location, user profile, topics) is usually missing
in a guideline statement. Enhancing the relevance
checking with contextual information would improve
the quality of the search results. In this paper, we pro-
pose a topic-centric approach to detect new evidence
for updating evidence-based medical guidelines as a
context-aware method to improve the search. We con-
sider the title of the section or subsection containing
a guideline statement as the topic of that guideline
statement. In the semantic distance based approach,
the terms appearing in the topic (i.e., in the title of the
section or subsection) should be ranked as more im-
portant than other terms. We have conducted several
experiments with this topic-centric approach to find
new relevant evidence for guideline updates. We will
show that this topic-centric approach indeed provides
a better result.
This paper is organized as follows: Section 2
introduces the basic structure of guidelines and the
procedure of guideline update, presents the approach
based on a semantic distance measure over terms, and
describes several strategies using the semantic dis-
tance measure for finding new and relevant evidence
for guidelines. Section 3 proposes the topic centric
approach. Section 4 presents several experiments of
our method on the update of guidelines. Section 5
discusses future work and concludes.
2 EVIDENCE-BASED
GUIDELINES AND GUIDELINE
UPDATES
2.1 Guideline Updates
Evidence-based medical guidelines are based on pub-
lished scientific research findings. Those findings are
usually found in medical publications such as those
in PubMed. Selected articles are evaluated by an ex-
pert for their research quality, and are graded for the
degree to which they contribute evidence using a clas-
sification system (NSRS, 2006).
A usual classification of research results in ev-
idence levels consists of the following five classes
(NSRS, 2006; NABON, 2012): Type A1: Systematic
reviews, or that comprise at least several A2 quality
trials whose results are consistent; Type A2: High-
quality randomised comparative clinical trials of suf-
ficient size and consistency; Type B: Randomised
clinical trials of moderate quality or insufficient size,
or other comparative trials (non-randomised, compar-
ative cohort study, patient control study); Type C:
Non-comparative trials, and Type D: Opinions of ex-
perts. Based on this classification of evidence, we
can classify the conclusions in a guideline (sometimes
called guideline items) with an evidence level. The
following evidence levels for guideline items are pro-
posed in (NABON, 2012): Level 1: Based on 1 sys-
tematic review (type A1) or at least 2 independent A2
reviews; Level 2: Based on at least 2 independent type
B reviews; Level 3: Based on 1 type A2 or B research,
or any level of C research, and Level 4: Opinions of
experts.
Here is an example of a conclusion in a guideline
in (NABON, 2012):
Classification: Level 1
Statement:
The diagnostic reliability of ultrasound
with an uncomplicated cyst is very high.
Evidence: A1 Kerlikowske 2003, B Boerner 1999,
Thurfjell 2002, Vargas 2004
which consists of a conclusion classification (’Level
1’), a guideline statement, and its evidence items with
one item classified as A1 and three items classified as
B (jointly justifying the Level 1 of this conclusion).
In order to check if there is any new evidence from
a scientific paper which is relevant to the guideline
A Topic-centric Approach to Detecting New Evidences for Evidence-based Medical Guidelines
283

statement, a natural way to proceed is to use the terms
which appear in the guideline statement to create a
query to search over a biomedical search engine such
as PubMed. In our experiments reported in (Hu et al.,
2015), we use Xerox’s NLP tool (Ait-Mokhtar et al.,
2013; A
¨
ıt-Mokhtar et al., 2002) to identify the med-
ical terms from UMLS and SNOMED CT which ap-
pear in guideline statements (Huang et al., 2014), and
then use these terms to construct a PubMed query to
search for relevant evidence. The resulting PubMed
ID (alternatively called PMID) can serve as the ID of a
retrieved evidence. A naive approach to creating such
a PubMed query is to construct the conjunction or dis-
junction of all terms that appear in a guideline item.
We have observed the following facts: i) the result
size of the conjunctive query often leads to 0 results
(67% of the cases), and ii) the result of the disjunctive
query would frequently lead to too many results (aver-
age 812,632, max 9,211,547) (Reinders et al., 2015).
The main problem of those approaches is that the se-
mantic relevance of the terms is not well considered.
An improved approach is to use a semantic distance
measure to create search queries in which more re-
levant terms are preferred to less relevant terms. In
other words, the semantic distance measure provides
us with a method to rank the terms in the search query.
The method consists of several steps that need to
be executed in order, as follows:
1. Extract the terms and the PMID of the evidences.
2. Use different terms ranking strategies.
3. Construct a PubMed query based on ranked terms.
4. Execute the query and evaluate the results.
5. Present the best results to the user.
In (Hu et al., 2015), we propose a semantic dis-
tance measure to rank terms for finding relevant ev-
idence from a Biomedical search engine such as
PubMed. Our semantic distance measure is based on
the (widely shared) assumption that more frequently
co-occurring terms are more semantically related.
In order to make this paper self-contained, we de-
scribe the relevant notions of the semantic distance
method in the following:
The equation for our Normalized PubMed Dis-
tance (NPD) is as follows:
NPD(x,y) =
max{log f (x),log f (y)} − log f (x,y)
logM − min{log f (x),log f (y)}
Where f (x) is the number of PubMed hits for the
search term x; f (y) is the number of PubMed hits for
the search term y; f (x, y) is the number of PubMed
hits for the search terms x and y; M is the number of
PMIDs indexed in PubMed (where M=23,000,000 at
the time of writing). NPD(x,y) can be understood in-
tuitively as the symmetric conditional probability of
co-occurrence of the search terms x and y (Cilibrasi
and M.B.Vitanyi, 2007).
Let G be a set of guideline statements and Terms
be the set of all terms. The function T : G →
Powerset(Terms) assigns a set of terms to each guide-
line statement such that T (g) is the set of terms which
appear in the guideline statement g. For each guide-
line statement g ∈ G and a term x in T (g), we define
AD(x,g) as the average distance of x to other terms in
g:
AD(x,g) =
∑
y∈T (g),y6=x
NPD(x,y)
|T (g)| − 1
We define the center term CT (g) as the term whose
average distance to other terms (in the guideline state-
ment g) is minimal:
CT (g) = arg
x
min(AD(x,g))
We can now consider the following different strate-
gies for term ranking:
• Average Distance Ranking(ADR): ranks the terms
by their average distance value.
• Central Distance Ranking(CDR): ranks the terms
by their distance to the center term, where the cen-
tral distance of a term x in a guideline statement
g, written as CD(x,g), is defined as:
CD(x, g) = NPD(x,CT (g))
We propose the following criteria the for evaluting
the results:
• Term Coverage Criteria: The more terms which
appear in the guideline statement are used for
search, the more relevant the results are;
• Evidence Coverage Criteria: The more original
evidences have been covered in the search, the
more relevant the results are;
• Bounded Number Criteria: It is not meaningful
to have too many results (for example more than
10,000 papers). Furthermore, we would likely
miss many evidence items if there are too few re-
sults (for example, less than 10 papers). Thus,
we can set the upper bound and lower bound of
the results. The former is called the upper bound
number P
u
, whereas the latter is called the lower
bound number P
l
.
Based on the three assumptions above, we design
a heuristic function f (i) to evaluate the search results
at each step at the workflow above:
f (i) = k
1
T (i)/T + k
2
E(i)/E + k
3
(P
u
− P(i))/P
u
HEALTHINF 2016 - 9th International Conference on Health Informatics
284

where T is the total number of terms in the guide-
line statement; T (i) is the number of selected terms
in this search i; E is the total number of the evidence
items for the guideline statement; E(i) is the number
of the original evidence items which has been cov-
ered in this search i; P
u
is the upper bound number;
P(i) is the number of PMID’s that result from this
search i, if P(i) is a number between P
u
and P
l
, and
k
1
, k
2
, k
3
are the weights of the different criteria. It
is easy to see that the first part of the heuristic func-
tion (e.g.,k
1
T (i)/T ) measures the Term Coverage Cri-
terion, the second part of the function (e.g., k
2
E(i)/E)
measures the Evidence Coverage Criterion, whereas
the third part of the function (e.g., k
3
(P
u
− P(i))/P
u
)
measures the Bounded Number Criterion with the
meaning that the fewer results are returned, the more
preferred they are (if the result size is between P
u
and
P
l
).
In (Hu et al., 2015), we have reported several ex-
periments to evaluate the above approach. We se-
lected the Dutch breast cancer guideline (version 1.0,
2004) (NABON, 2004) and the Dutch breast cancer
guideline (version 2.0, 2012) (NABON, 2012) as the
test data. From these experiments, we found that there
is room to improve the search results by reducing the
sizes of the returned results and to find more goal ev-
idence for more guideline items. In (Hu et al., 2015)
and as explained above, the center term is defined as
the term for which the average distance to other terms
is minimal. That definition of center term is inde-
pendent of the topic of a selected guideline conclu-
sion (where by topic, we mean the titles of the sec-
tions or subsections in which the guideline conclu-
sions are contained). An intuitive approach is to se-
lect the terms which appear in the topic to be a center
term. The contribution of this paper is to develop this
topic-centric approach to find new evidence. We will
report the experiments that compare the non-topic-
centric approach with the topic-centric approach in
Section 4.
3 TOPIC-CENTRIC APPROACH
FOR FINDING NEW
EVIDENCES
Contextualization has been considered to be a useful
approach to improve the quality of search, because
the context can provide more precise information for
users to make queries and to reduce the size of search
results (Stalnaker, 1999). Typically, spatial and tem-
poral information about the users and the systems are
considered as contextual information, because they
are usually not stated explicitly when users make a
search. Personalization can be also considered as a
special case of contextualization. The same scenario
can be also applied to the topic that the search is con-
cerned with, since this is usually also not stated ex-
plicitly.
For medical guidelines, it is quite convenient to
obtain this topic information, because each guideline
recommendation or conclusion is always covered in
a section or a subsection with a specific title. Of
course, the title of a section or a subsection may con-
tain multiple terms. Again we can use the semantic
distance measure to rank the terms appearing in the
topic. Therefore, a topic centric approach to rank the
terms can be done as follows:
1. Obtain the terms which appear in the title of sec-
tion or subsection of a guideline conclusion. They
are called the topic terms.
2. Rank the topic terms by using the semantic dis-
tance measure. The first term in the ranking is
considered to be the center term.
3. Add non-topic terms which appear in the guide-
line statement one by one, based on their semantic
distance to the center term.
4. Create a search query based on the merged set of
the topic terms and non-topic terms.
5. Search over PubMed to find relevant evidence by
using the generated queries.
6. Select the best query answer based on the heuris-
tic function.
Let G be a set of guideline statements and Terms
be the set of all terms. The function Topic : G →
Powerset(Terms) assigns a set of terms to each guide-
line statement such that Topic(g) is the set of terms
which appears in the title of the section or the sub-
section in which the guideline statement g appears.
Of course, the intersection of the terms and the topic
terms of a guideline statement may not be an empty
set:
T (g) ∩ Topic(g) 6=
/
0
In the topic-centric approach, for each guideline state-
ment g ∈ G and term x in Topic(g), we can define
the average distance of term x ∈ Topic(g), written as
AD
T
(x,g) as follows:
AD
T
(x,g) =
∑
y∈Topic(g),y6=x
NPD(x,y)
|Topic(g)| − 1
We define the center term CT
T
(g) in the topic as the
term whose average distance to other terms (in the
guideline statement g) is minimal:
CT
T
(g) = arg
x
min(AD
T
(x,g))
A Topic-centric Approach to Detecting New Evidences for Evidence-based Medical Guidelines
285
In this paper, we use the strategy of the Central Dis-
tance Ranking with the topic. Namely, this strat-
egy ranks the topic terms by their distance to the
center term in the topic first, then ranks those non-
topic terms by their distance to the center term in the
topic, where the central distance of a term x ∈ T (g)
in the topic for a guideline statement g, written as
CD
T
(x,g), is defined as:
CD
T
(x,g) = NPD(x,CT
T
(g))
4 EXPERIMENTS AND
EVALUATION
We have implemented the guideline update tool as
a component in SemanticCT, a semantically-enabled
system for clinical trials (Huang et al., 2013; Hu et al.,
2014)
4
. We have conducted several experiments for
finding relevant evidence for guideline updates. We
selected the Dutch breast cancer guideline (version
1.0, 2004) (NABON, 2004) and the Dutch breast can-
cer guideline (version 2.0, 2012) (NABON, 2012)
as test data. For our experiments we have selected
16 conclusions which appear in both versions of the
guidelines. Thus, the evidence items appearing in the
second version of the guideline can serve as a gold
standard to test the proposed approach in this paper.
Namely, we want to know whether or not finding re-
levant evidence for the first version of the guideline
can really find the target evidence (alternatively called
goal evidence items) which was used on the second
version of the guideline. For the non-topic-centric ap-
proach, one of our experiments in (Hu et al., 2015) is
using the Central Distance Ranking. We compare the
results of the topic centric approach with the results of
the non-topic centric approach to see whether or not it
can get a better result, namely, finding goal evidence
items for more guideline statements.
Our first experiment is to use the topic centric ap-
proach to find relevant evidence for the sixteen se-
lected guideline conclusions and use the same heuris-
tic function to guide the search with the same weights
on the three criteria, namely k
1
= k
2
= k
3
= 1/3 and
the upper bound number P
u
= 1000 and the lower
bound number P
l
= 25. The results of a comparison
the non-topic-centric approach are shown in Table 1.
In this experiment, the topic centric approach can find
the goal evidence items for 12 guideline statements
out of 16 ones. Compared with the results obtained by
using the non-topic centric approach (which can find
goal evidences for only 5 guideline statements), it gets
4
http://wasp.cs.vu.nl/sct
Figure 1: Systematic Tests on Different Weights.
a much better result. We have also observed that it
is not always the case that the topic-centric approach
gives a better result. For example, for the guideline
statement 04 1 3, the non-topic-centric approach can
find 3 goal evidence items, whereas the topic-centric
approach can find only one. This increase in items
for which any goal evidence is found (12 against 5)
comes at the price of a slightly lower percentage of
evidence items found per case (dropping from 57%
to 46%). This small drop is well worth the steep in-
crease from 5 to 12 cases for which any goal evidence
is found. As a result, across the entire set of guideline
items the topic-centered approach scores better (41%
against 18%).
Unfortunately, we have observed that for guide-
line statements with non-zero goal-items, the result
sizes of the topic-centric approach are larger. The ap-
parent large difference (1089 against 135.4) is partly
caused by an abnormally big size coming from guide-
line statement 04 6 1. An explanation why this guide-
line statement leads to such as large result size is that
it can find all of the five goal evidences. Thus, the Ev-
idence Coverage Criterion overwhelms the Bounded
Number Criteria. When removing this outlier, the dif-
ference drops to 287 against 135.4). A similar pattern
holds for the overall result sizes.
In the experiment above, we used equal weights
for the three criteria, i.e., k
1
= k
2
= k
3
= 1/3. The
next experiments make a systematic test through dif-
ferent combinations of weights to see which weight
values would provide the best results. We consider
the following five possible values of the weights to
cover the 0-1 interval:
{0,0.25.0.5,0.75,1}
for a single criterion weight.
HEALTHINF 2016 - 9th International Conference on Health Informatics
286
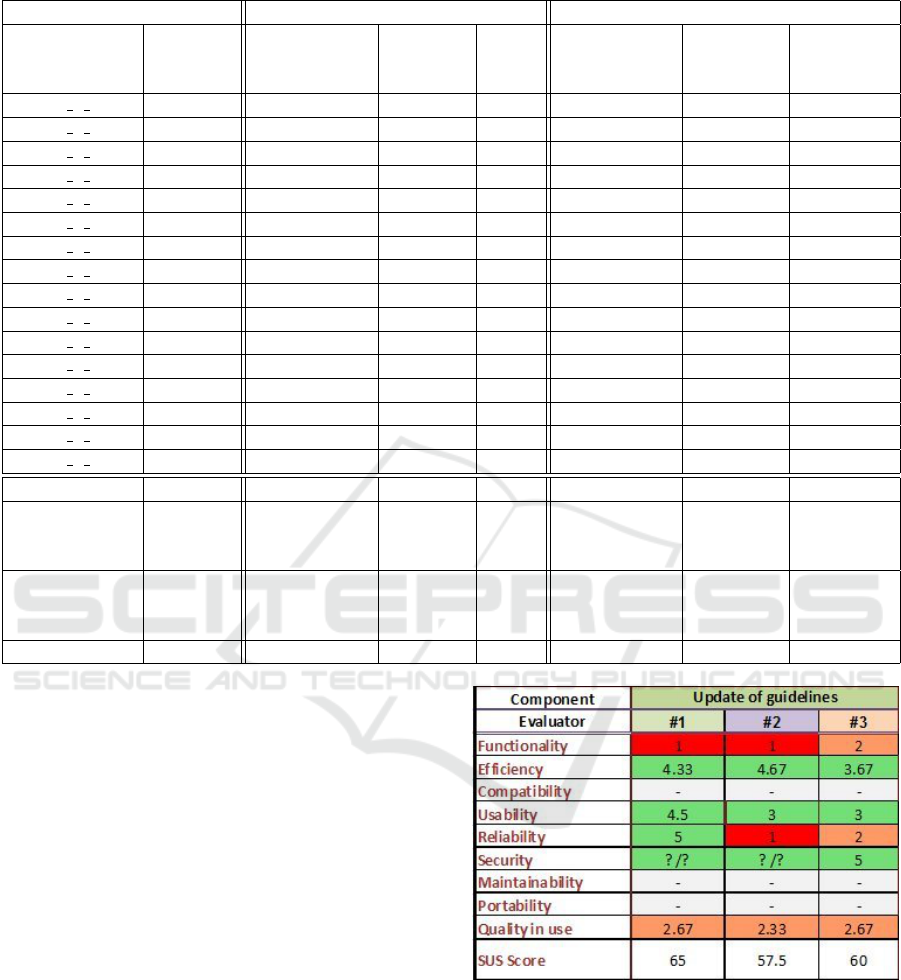
Table 1: Comparison between topic-centric approach and non-topic-centric approach with k
1
= k
2
= k
3
= 1/3.
Non-topic-centric Topic-centric
Original Found Found Found Found
ID Evidence Goal Evidence % Goal Evidence %
Number Evidence No. Number Evidence No. Number
04 1 1 5 1 69 20% 2 60 40%
04 1 2 2 1 60 50% 1 166 50%
04 1 3 4 3 327 75% 1 36 25%
04 3 1 4 0 89 0% 0 49 0%
04 3 2 2 2 62 100% 1 28 50%
04 3 3 2 0 27 0% 0 33 0%
04 3 5 2 0 39 0% 1 333 50%
04 3 6 8 3 159 38% 3 140 38%
04 3 7 2 0 52 0% 1 89 50%
04 4 1 5 0 219 0% 3 1628 60%
04 4 2 5 0 42 0% 3 281 60%
04 5 1 3 0 77 0% 0 82 0%
04 6 1 5 0 62 0% 5 9911 100%
04 6 2 3 0 89 0% 1 72 33%
04 7 1 2 0 9 0% 0 372 0%
04 8 1 2 0 15 0% 1 324 50%
Total 56 10 1397 18% 23 13604 41%
No.
of non-zero 5 12
goal evidences
Average
for non-zero 135.4 57% 1089(287) 46%(41%)
goal evidences
Average 3.5 87 850(230)
Because of the normalization condition of the
three criteria weights (i.e., k
1
+k
2
+k
3
= 1), once two
weights are fixed (say, k
1
and k
2
), the third weight is
also fixed (i.e., k
3
= 1 − k
1
− k
2
). Thus, the possi-
ble combinations of the weights can be considered as
a two-dimensional table with k
1
= 0, 0.25.0.5.0.75, 1
and k
2
= 0,0.25,0.5,0.75,1 respectively.
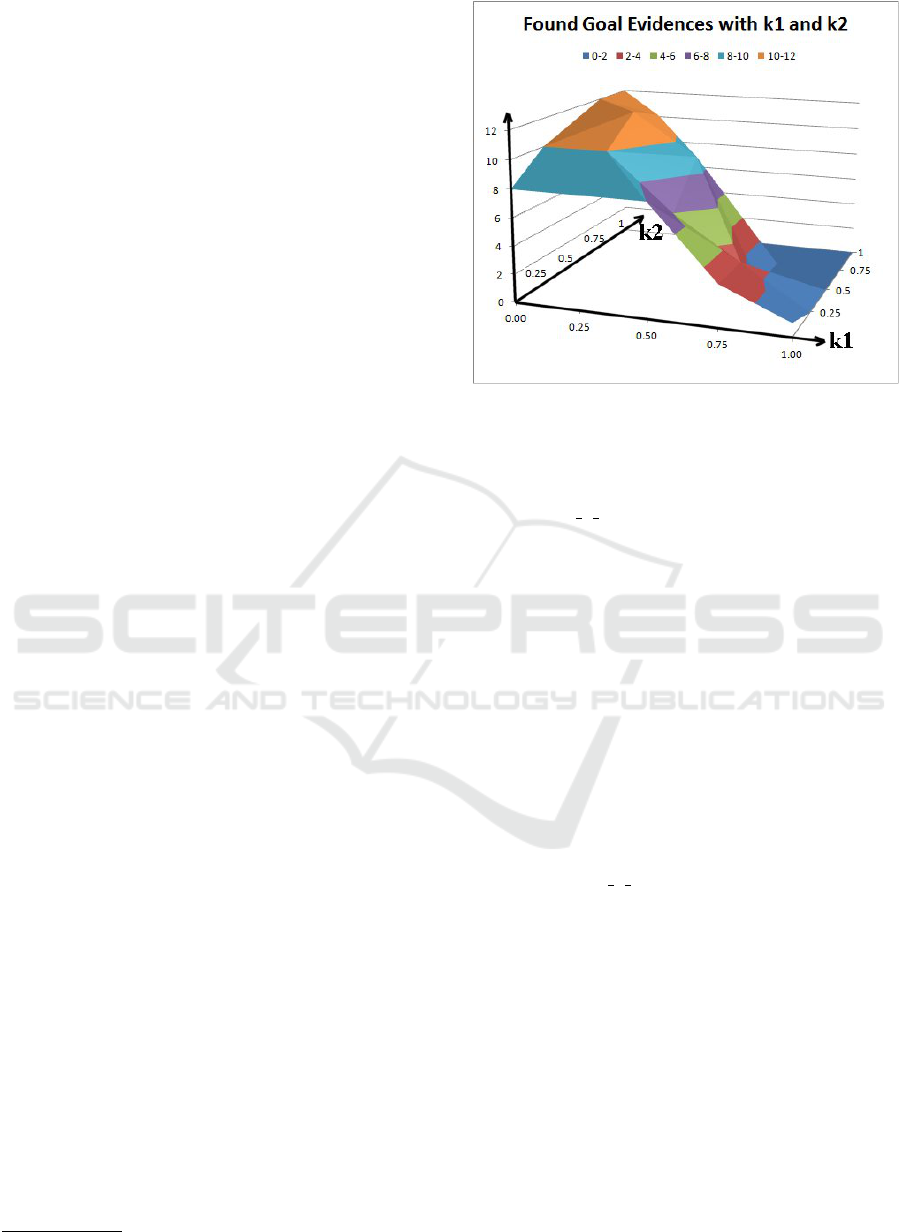
Figure 1 shows how many guideline items can find
their goal evidence when the weights k
1
and k
2
are set
to different values. From the systematic tests with dif-
ferent value combinations, we can see that the system
achieves better results when k
2
is set to higher values
(i.e., 0.75 or 1). This means that the second crite-
rion (i.e., to check how much of the original evidence
which has been used in the current version are cov-
ered in the search) plays the most important role on
getting better results.
In order to evaluate the proposed approach, we
invited three medical professionals from the MAAS-
TRO clinic in the Netherlands to score the guideline
update tool with respect to various properties such
as functionality, efficiency, usability, reliability and
quality of use. The evaluation results are shown in
Figure 2, where all the properties are measured on a
Figure 2: Evaluation by Medical Professional. Scale: 1
(worst) to 5 (best).
scale from 1 (worst) to 5 (best).
The main conclusions from this evaluation by
medical professionals are: i) The tool has potential
to save time and to identify new relevant evidence for
experts who are updating guidelines, ii) There are still
too many irrelevant articles suggested as evidence.
This produced an overwhelming number of irrelevant
articles and reduced the evaluator’s overall confidence
A Topic-centric Approach to Detecting New Evidences for Evidence-based Medical Guidelines
287

in the tool. From that evaluation, we know that the
next big step is to improve the precision of the search
process
5
.
5 CONCLUSION AND FUTURE
WORK
In this paper, we have presented a topic-centric ap-
proach for searching over new and relevant evidence
for updating medical guidelines. We have reported
several experiments of the proposed approach and
compared our results with those of the non-topic cen-
tric approach. The experiments show that the topic
centric approach can find goal evidence items for 12
guideline statements out of 16, while the non-topic
centric approach can find goal evidence items for only
5 guideline statements. Across the entire corpus of
guideline items, the percentage of found goal eviden-
ces doubles from 18% to 41%.
Compared with the results of Reinders’ approach
(Reinders et al., 2015) (with an average result
size over one million), the result sizes in our ap-
proaches are much smaller. Our approaches are dif-
ferent from Iruetaguena’s approach (Iruetaguena et al,
2013), which relies on gathering all relevant articles
by searching the PubMed website. Our semantic-
distance-based approach can gain a better perfor-
mance (an average of approximately 10 minutes for
each guideline statement) (Hu et al., 2015). There are
no differences in the runtime between the non-topic
centric approach and the topic centric approach, be-
cause adding topic terms in the ranking does not lead
to any expensive computation.
There is still future work to improve the existing
methods. For example, we can introduce an ontology-
based semantic distance measure, so that two seman-
tically equivalent concepts in a medical terminology
(says SNOMED CT or UMLS) can be considered to
have a zero semantic distance. Thus, relevance mea-
sure can be independent from two terms, but instead
only depends on the underlying semantic concepts.
Another approach to improve the result ranking is to
consider the journal classes of the evidence. We can
always prefer a publication which appears in a top
journal. In future work, we will also do an extensive
second evaluation on more medical guidelines.
5
The software, as well as all exper-
imental data and results is available at
http://wasp.cs.vu.nl/sct/download/release/GuidelineUpdate
Tool-v0.7.zip
ACKNOWLEDGEMENTS
This work is partially supported by the European
Commission under the 7th framework programme
EURECA Project (FP7-ICT-2011-7, Grant 288048).
We thank the clinical trial experts in the MAASTRO
clinic for their help on the evaluation.
REFERENCES
Ait-Mokhtar, S., Bruijn, B. D., Hagege, C., and Rupi, P.
(2013). Initial prototype for relation identification be-
tween concepts, D3.2. Technical report, EURECA
Project.
A
¨
ıt-Mokhtar, S., Chanod, J.-P., and Roux, C. (2002). Ro-
bustness beyond shallowness: incremental deep pars-
ing. Natural Language Engineering, 8(2):121–144.
Cilibrasi, R. and M.B.Vitanyi, P. (2007). The google simi-
larity distance. IEEE Trans. Knowledge and Data En-
gineering, 19:370–383.
Hu, Q., Huang, Z., den Teije, A., and van Harmelen, F.
(2015). Detecting new evidence for evidence-based
guidelines using a semantic distance method. In Pro-
ceedings of the 15th Conference on Artificial Intelli-
gence in Medicine(AIME 2015).
Hu, Q., Huang, Z., van Harmelen, F., ten Teije, A., and
Gu, J. (2014). Evidence-based clinical guidelines in
SemanticCT. In The Semantic Web and Web Sci-
ence, Volume 480 of the series Communications in
Computer and Information Science, pages 198–212.
Springer.
Huang, Z., ten Teije, A., and van Harmelen, F. (2013). Se-
manticCT: A semantically enabled clinical trial sys-
tem. In Lenz et al., R., editor, Process Support and
Knowledge Representation in Health Care. Springer
LNAI.
Huang, Z., ten Teije, A., van Harmelen, F., and Ait-
Mokhtar, S. (2014). Semantic representation of
evidence-based clinical guidelines. In Proceedings of
6th International Workshop on Knowledge Represen-
tation for Health Care (KR4HC’14).
Iruetaguena et al, A. (2013). Automatic retrieval of current
evidence to support update of bibliography in clinical
guidelines. Expert Sys with Apps, 40:2081–2091.
NABON (2004). Guideline for the treatment of breast carci-
noma 2004. Technical report, Nationaal Borstkanker
Overleg Nederland (NABON).
NABON (2012). Breast cancer, dutch guideline, ver-
sion 2.0. Technical report, Integraal kankercentrum
Netherland, Nationaal Borstkanker Overleg Neder-
land.
NSRS (2006). Guideline complex regional pain syndrome
type i. Technical report, Netherlands Society of Reha-
bilitation Specialists.
Reinders, R., ten Teije, A., and Huang, Z. (2015). Finding
evidence for updates in medical guideline. In Pro-
ceedings of HEALTHINF2015. Lisbon.
HEALTHINF 2016 - 9th International Conference on Health Informatics
288

Stalnaker, R. (1999). Context and content. Oxford: Oxford
University Press.
Woolf, S., Grol, R., Hutchinson, A., Eccles, M., and
Grimshaw, J. (1999). Clinical guidelines:potential
benefits, limitations, and harms of clinical guidelines.
BMJ, 318(7182):527–530.
A Topic-centric Approach to Detecting New Evidences for Evidence-based Medical Guidelines
289
