
A Targeting Self-breakable Agent for Increased Efficacy of
Chemotherapeutic Drugs against Caco2 Cells
Ming-Hsien Tsai
1
, Ming-Jium Shieh
1
and Cheng-Liang Peng
2
1
Institute of Biomedical Engineering, National Taiwan University College of Medicine, National Taiwan University,
No1, Sec. 1, Jen - Ai Rd., Taipei, Taiwan
2
Isotope Application Division, Institute of Nuclear Energy Research, No.1000, Wenhua Rd., Longtan Dist., Taiwan
Keywords: SN38, Micelle, Caco2.
Abstract: Many types of nano-sized anti-cancer agents that could increase efficacy of chemotherapeutic drugs have
been created and developed in colon cancer treatment over years. Moreover, with the intention of achieving
the ideal chemotherapeutic efficacy, nano-sized anti-cancer agents were further designed to have specific
functions, efficiently killing colon cancer cells. Our research team focused on two important functions in
designing nano-sized agents, controlled drug release and targeting functions. Thus, targeting functional
micelles which entrapped chemotherapeutic drug, 7-ethyl-10-hydroxy-camptothecin (SN38) were designed
in nano-size and possessed disulfide bonds in this study. In particular, Self-Breakable SN38-loaded micelles
(SN/38 micelles), Non-Breakable micelles SN38-loaded (NB/38 micelles) and Folate-targeting Self-
Breakable SN38-loaded micelles (FSB/38 micelles) were prepared and tested to the designed agents. The
results showed that the folate-decorated functional micelles with disulfide bonds could be an effective
chemotherapeutic agent for colon cancer treatment.
1 INTRODUCTION
Chemotherapy is the most common therapy for
colorectal cancer, which is an intractable issue for
human beings due to its increased chance of death so
cancer studies are still ongoing for developing high-
efficacy chemotherapeutic drugs(Bala et al., 2013).
A good candidate for drug agents is micelle
composed of amphiphilic polymers with nanoscale
size allowing accumulation of the micelles in the
tumor through enhanced permeability and retention
(EPR) effect, thereby resulting in high tumor
uptake(Joralemon et al., 2010). However, the
efficacy of chemotherapeutic drug-loaded micelles
without specific functions is unsatisfactory.
Recently, the designed micelles with specific
functions, for instance, targeting function(Xu et al.,
2013), photodynamic function(Peng et al., 2008),
and controlled release function(Peng et al., 2010,
Peng et al., 2011b) have been created. Such
functional micelles were shown to have greater
effectiveness in cancer treatments(Sinn Aw et al.,
2014).
Folate which has exhibited outstanding ability in
increasing cellular uptake of the loaded drug was
chosen as the targeting ligands in this study (Khatik
et al., 2015, Cuong et al., 2012). In addition, unlike
many other targeting ligands used in
chemotherapeutic drugs, folate, is safe for human
consumption, and has approved by the US Food and
Drug Administration for the use in dietary
supplements. Based on these reasons, folate is a
good choice for a targeting ligand which can be used
to modify micelles, which can efficiently increase
the cellular uptake and efficacy of the
chemotherapeutic drug.
However, even if the folate-decorated functional
micelles increase cellular or tumor uptake of
functional micelles, the entrapped drug might fail to
release owing to its rigid structure, which results in
its lower efficacy(Xing et al., 2015). The folate-
decorated functional micelles in this study were
further designed for successful drug release which
plays an important role in achieving optimal efficacy
of chemotherapeutic drugs. This is attributed to the
fact that drug must reach drug action site in tumor
cell for drug action to take place(Kawato et al.,
1991). Great efforts have been made to create
controlled release micelles which are self-breakable,
particularly redox-responsive functional micelles,
216
Tsai, M-H., Shieh, M-J. and Peng, C-L.
A Targeting Self-breakable Agent for Increased Efficacy of Chemotherapeutic Drugs against Caco2 Cells.
DOI: 10.5220/0005766002160221
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 1: BIODEVICES, pages 216-221
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

for the enhancement of the drug efficacy. Disulfide
bonds created in such functional micelles quickly
react with glutathione (GSH) which renders the
micelles unstable, thereby enabling them to release
the drug spontaneously (Huo et al., 2014, Lai et al.,
2014). Therefore, the folate-decorated functional
micelles created in this study were designed to be
redox-responsive.
We attempted to enhance efficacy of the
chemotherapeutic drug, 7-Ethyl-10-hydroxy-
camptothecin (SN38), an active metabolite of the
clinical drug, irinotecan (CPT-11) which is used in
the treatment of colorectal cancer. For this purpose,
Folate-targeting Self-Breakable micelles (FSB
micelles) consisting of self-degradable copolymers,
and targeting copolymer, were created to setup an
active drug delivery system using a colorectal cancer
cell line, Caco2. FSB micelles could facilitate Caco2
in acidic microenvironment to take up the loaded
SN38, resulting in enhanced drug efficacy. In
addition, to confirm that FSB micelle can be the best
anti-cancer agent, Self-Breakable micelles (SB
micelles) without targeting function and Non-
Breakable micelles (NB micelles) which have no
specific function were also created to evaluate the
designed functions of FSB micelles.
2 EXPERIMENTAL SECTION
2.1 Synthesis of the Self-degradable,
Non-self-degradable Copolymers
and Targeting Copolymers
The non-self-degradable (ND) copolymers,
methoxyPolyEthylene Glycol-PolyCaproLactone,
(mPEG-PCL) was synthesized as described in our
earlier research(Chen et al., 2015). The self-
degradable (SD) copolymers, methoxyPolyEthylene
Glycol-S-S-PolyCaproLactone (mPEG-S-S-PCL)
was primarily obtained via two chemical reactions.
MPEG-SH was reacted with excess 2-
mercaptoethanol in deionized water to obtain
mPEG-S-S-C
2
H
4
OH. Then, MPEG-S-S-PCL was
obtained via the ring-opening polymerization.
To prepare the targeting copolymer, Folate-
Poly(Ethylene Glycol)-Poly(CaproLactone) (F-PEG-
PCL), FMOC-NH-PEG-PCL was used to synthesize
as described (Peng et al., 2011a). F-PEG-PCL was
obtained by conjugating the de-protected polymer,
NH
2
-PEG-PCL, with folate via an amide bond. The
designed copolymers were characterized by
1
HNMR, FT-IR, and Gel Permeation
Chromatography (GPC) was used to determine the
molecular weight (MW) of copolymers.
2.2 Characteristics of Self-breakable,
Non-self-breakable, and Targeting
Self-breakable Micelles
The NB micelles prepared from ND copolymers and
SB micelles prepared from SD copolymers in this
study were prepared to evaluate the function of SB
micelles in triggering the release of loaded drug in
the presence of GSH in cancer cells. The FSB
micelles prepared using a mixture containing 80%
(w/w) SD and 20% (w/w) targeting copolymers were
designed to have targeting and self-breakable
function for achieving the best chemotherapeutic
efficacy in cancer treatment.
SB micelles, NB/38 micelles, SB/38 micelles,
and FSB/38 micelles were prepared using a
lyophilization-hydration method. The SN38-loaded
micelle formulations containing 10mg/mL of
polymer and 1mg/mL of SN38 in PBS were filtered
using 0.22µm filter to remove non-loaded SN38.
Then, the size of micelles was determined by
Transmission Electron Microscopy (TEM), and
Dynamic Light Scattering (DLS). Loading
Efficiency (LE) and Drug Content (DC) were
determined using the calibration curve based on
maximum absorption values of SN38 in DMSO.
Critical Micelle Concentration (CMC) of the
micelles was determined using pyrene as described
elsewhere.
2.3 Physical and Chemical Stability of
the Micelles
To access whether SB/38 micelles, and NB/38
micelles were self-breakable agents or not, the
micelles were incubated with or without 10mM DTT
in phosphate buffered saline (PBS) which was used
to simulate glutathione (GSH) in cells. Incubation
with or without DTT was conducted at 37°C over
specific time periods. Then, the micelle stability was
determined by their size and polydispersity (PdI). At
select time points, the size and PdI of the micelles
were determined by DLS.
To prove that disulfide bonds designed in the SD
copolymers could be broken up by GSH in the cells,
the MW of copolymers self-assembling into SB
micelles were analysed via GPC after the incubation
with 10mM DTT. In brief, SB/38 micelles were
lyophilized after incubation at 37°C for 24 h. The
lyophilized SB/38 micelles were dissolved in THF,
A Targeting Self-breakable Agent for Increased Efficacy of Chemotherapeutic Drugs against Caco2 Cells
217

and then the MW of the polymers of SB/38 micelles
was determined via GPC.
2.4 Drug Release Profile
In vitro SN38 release profiles of SB/38 and NB/38
micelles, dispersed in PBS with or without DTT,
were analysed using a modified dialysis-bag
diffusion technique at 37°C. The dialysis tube
containing 0.4 mL of the micelle formulation was
suspended in 100 ml PBS in a closed bottle. A
magnetic mixer was introduced into the bottle and
incubated at 37°C. Every 1ml of aliquot was
withdrawn from the external media and refilled with
1ml of fresh PBS at select time intervals. The SN38
concentration was determined by fluorescence
intensity at 427nm (excitation at 390nm). All
experiments were conducted in triplicate.
2.5 In Vitro Cytotoxicity
The human colon cancer cell line, Caco2, was
cultured in a humidified 5% CO
2
incubator at 37 °C
in Minimum Essential Media, MEM (GIBCO BRL,
Gaithersburg, MD, USA) supplemented with 20%
heat-activated fetal bovine serum (FBS), 1% non-
essential amino acids, 2 mM L-glutamine, 1 mM
sodium pyruvate, 1500 mg/L sodium bicarbonate,
and 1% (v/v) Penicillin-Streptomycin Amphotericin
B Solution (GIBCOBRL). Initially, the Caco2 cells
were seeded onto 96-well plates at a density of
10,000 cells per well and cultured. After 24 h, cells
were incubated in media containing different
concentrations of SN38 for 6h. Then, the cells were
washed three times with PBS to remove the
suspended SN38 and cultured with fresh medium for
another 48 h. Cell viability was assessed using MTT
assay with a scanning multi-well ELISA reader
(Microplate Autoreader EL311, Bio-Tek Instruments
Inc., Winooski, VT, USA). The cytotoxicities of SB
micelles, NB micelles, FSB/38 micelles, SB/38
micelles and NB/38 micelles were also evaluated by
the same method.
Scheme 1: Structure of a targeting self-breakable drug-
loaded micelle.
3 RESULTS AND DISCUSSION
3.1 Synthesis of the Self-degradable,
Non-self-degradable Copolymers
and Targeting Copolymer
The FSB micelles composed of SD copolymers and
targeting copolymers were used as a targeting self-
breakable agent for the enhancement of drug
efficacy and were loaded with the chemotherapeutic
drug, SN38, used to treat colon cancer in this study
(scheme 1). The
1
H-NMR results revealed that SD
copolymer was successfully synthesized and had a
MW of 8,530 g/mol (data not shown). GPC analysis
indicated SD copolymer had a molecular weight,
11,253 g/mol, and a narrow PolyDispersity (PD) of
1.15 (Table 1). The FT-IR spectra showed the
linkage of NH
2
PEG-PCL with folate via an amide
bond which indicated the successfully synthesis of
F-PEG-PCL (Figure 1).
3.2 Characteristics of Self-breakable,
Non-self-breakable, and Targeting
Self-breakable Micelles
The characteristics of the SB/38, NB/38 and FSB/38
are shown in Table 2. In terms of the size, the size of
NB/38 micelles, NB/38 micelles, and FSB/38
micelles were determined to be all about 130nm at
10:1 ratio of polymer/drug in PBS. The TEM images
further supported this finding as the results show
that the actual sizes FSB/38 micelles used as a
targeting self-breakable agent were the same as that
determined via DLS (Figure 3). Comparison of the
in vitro and in vivo test results involving the use of
each of these three SN38-loaded micelles ruled out
the possible issues associated with the difference in
size, perhaps due to their similarity in size. In
addition, owing to their uniform nano-size, these
SN38-loaded functional micelles could successfully
accumulate in the tumor via the EPR effect and thus
the enhance efficacy of drug, SN38.
Figure 1: FT-IR spectra of folate-PEG-PCL and folate.
mPEG-SS-PCL
F-PEG-PCL
SN38
Wavenumber(cm
-1
)
800 1000 1200 1400 1600 1800 2000 2200
Absorbance
Folate
F-PEG-PCL
1550
1651
1605
1740
1114
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
218

Table 1: Molecular characteristics of mPEG-S-S-PCL,
mPEG-5,000, and SB/38 micelles incubated with DTT
for 24 h.
Time (min)
16 18 20 22 24 26
mPEG-S-S-PCL
mPEG-5000
mPEG-S-S-PCL + DTT
Figure 2: GPC elugram of mPEG-S-S-PCL, mPEG-5,000,
and SB/38 micelles incubated with DTT for 24 h.
3.3 Physical and Chemical Stability of
the Micelles
To prove that SB micelles can be a self-breakable
agent, the SB/38 micelles, and NB/38 micelles were
incubated with DTT which was used to simulate
GSH with thiol groups in cells. SB/38 micelles with
DTT became larger than those without DTT over
time (Figure 4A). The PdI data shown in Figure 4B
indicated that SB/38 micelles with DTT had a wide
range of particle distribution (PdI : over 0.3) 3h after
the start of the test. As expected, these results were
due to the thiol groups in DTT reacting with
disulfide bonds in SB/38 micelles, causing SB/38
micelles to be relatively unstable and aggregate. In
contrast, the presence of DTT did not affect the
stabilities of NB/38 micelles during the course of the
whole experiment. Compared with SB/38 micelles,
NB/38 micelles remained stable.
Figure 3: TEM image of FSB/38 micelle.
Figure 4: Stability of SB/38 micelles or NB/38
micelles incubated with or without DTT for 24h was
determined by DLS in terms of size (A) and PdI (B).
Moreover, the GPC analysis (Table 1 and Figure
2) was performed to confirm that SB micelles
composed of mPEG-S-S-PCL could be a self-
breakable agent indicated that the disulfide bonds
designed in SB copolymers were broken up by DTT
after incubation of the SB/38 micelles with DTT,
resulting in a significant decrease in molecular
weight.
3.4 Drug Release Profile
Drug release profiles of SB/38 and NB/38 micelles
with or without DTT were conducted to determine
the micelle’s ability to release drug. As shown in
Figure 5, only SB/38 micelles successfully released
SN38 with DTT over time. DTT reacted with the
disulfide bonds in SB/38 micelles, which resulted in
a significant drug release. In contrast, the other
micelles released little amount of SN38 with or
without DTT (i.e., < 5% of SN38 released) over 96h.
This implies NB/38 micelles were relatively stable
regardless of the presence or absence of DTT. These
results are in accord with those obtained via DLS
and GPC informed us that unstable SB/38 micelles
will release drug. This proved again the efficacy of
SB/38 micelles to be used a potent drug for colon
cancer treatment.
Table 2: Characteristics of NB/38 micelle, SB/38
micelle, and FSB/38 micelle.
3.5 In Vitro Cytotoxicity
To evaluate the cytotoxicity of the free drug, the
designed nano-sized agents, free SN38, SB micelles,
NB micelles, SB/38 micelles, NB/38 micelles and
FSB/38 micelles were incubated with Caco2 cell
Copolymer/micelle Mn Mw Mp P.D.
mPEG-S-S-PCL 9,742 11,253 13,699 1.15
mPEG-5000 6,679 7,015 7,606 1.05
SB/38 micelles
(mPEG-S-S-PCL)
+10mM DTT
7,115 7,792 7,569 1.08
Time (h)
0 5 10 15 20 25 30
Size (nm)
0
1000
2000
3000
4000
5000
6000
7000
NB/38 micelle
NB/SN micelle + DTT 10mM
SB/SN micelle
SB/SN micelle + DTT 10mM
Time (h)
0 5 10 15 20 25 30
PdI
0.0
0.2
0.4
0.6
0.8
1.0
1.2
NB/38 micelle
NB/38 micelle + DTT 10mM
SB/38 micelle
SB/38 micelle + DTT 10mM
AB
Micelle Size (nm)
a
PdI
a
LE(%)
b
DC(%)
c
CMC
d
(wt%)
NB/38
130.0±2.1 0.14±0.03 94±4.3 8.6±0.21
0.0021
SB/38
132.4±1.2 0.10±0.04 93±4.1 8.5±0.13
0.0025
FSB/38
131.5±2.3 0.13±0.02 92±3.9 8.4±0.24
0.0023
A Targeting Self-breakable Agent for Increased Efficacy of Chemotherapeutic Drugs against Caco2 Cells
219
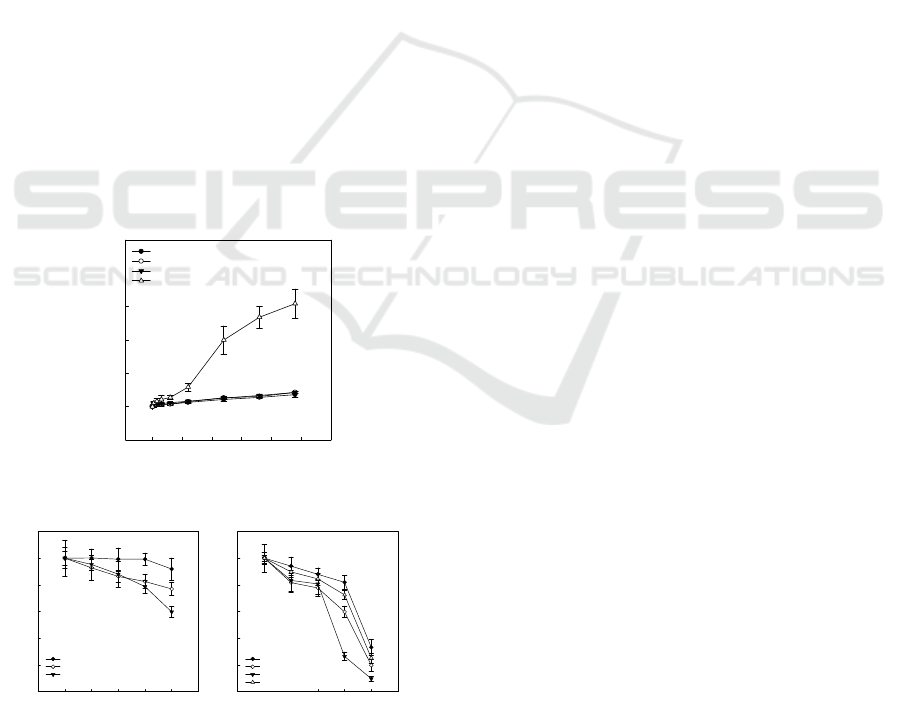
under conditions mimicking in vivo tumor
environment at a low pH(Vaupel et al., 1989,
Estrella et al., 2013). No toxicity was observed over
24 h in NB micelles and SB micelles (data not
shown), which confirmed that SB micelles could be
nontoxic owing to their biocompatibility. Free SN38
achieved the highest efficiency in killing cancer cells,
which was expected in this study, as it is known to
be the most toxic in vitro in cellular experiments
(Figure 6B). However, it is not clinically used.
Among the designed anti-cancer drugs without a
targeting function, the toxicity of SB/38 micelles
was significantly higher than that of NB/38 micelles
which was due to their successful self-controlled
drug release. Regarding to FSB/38 micelles, it was
found that they were able to achieve the highest
level of effectiveness in killing cancer cells among
the anti-cancer drugs studied. This can be attributed
to the fact that FSB/38 micelles had decorated-folate
on their surface which caused the Caco2 cells to take
up more FSB/38 micelles, resulting in the much
higher efficacy of SN38. In addition, to evaluate the
effect of medium pH on the cytotoxicity of FSB/38
micelles, a comparison of the cytotoxicity of FSB/38
micelles at a medium pH of 7.4 with that of FSB/38
micelles at a medium pH of 6.7 or 6 was conducted.
The highest cytotoxicity of FSB/38 micelles was
observed at pH 6 (Figure 6A). This could be
Time
(
h
)
0 20 40 60 80 100 120
Drug release (%)
-10
0
10
20
30
40
50
NB/38 micelle
NB/38 micelle + DTT 10mM
SB/38 micelle
SB/38 micelle + DTT 10mM
Figure 5: SN38 release profile.
Figure 6: Cell viability of FSB/38 micelles was evaluated
in media with different pH values, pH = 6, 6.7, and 7.4 (A).
Cell viability of SN38, NB/38 micelles, SB/38 micelles,
and FSB/38 micelles (B).
attributed to the fact that Caco2 cells quickly took up
folate in the medium at low pH, resulting in more
uptake of the folate-decorated FSB/38 micelles.
These results show that FSB/38 micelles could be an
effective drug for colon cancer treatment.
4 CONCLUSIONS
In this study, the nano-micelles were designed to be
self-breakable micelles, called SB/38 micelles. The
intention of creating SB/38 micelles was to improve
the drug release and enhance drug efficacy. The
results of DLS and GPC prove that SB/38 micelles
disassembled with DTT which was used to simulate
GSH with thiol groups in the cells, resulting in drug
release. Furthermore, the release profiles showed
that not only SB/38 micelles successfully released
SN38, but also a great amount of SN38 was released
with DTT. To effectively kill cancer cells and thus
ensure better results of cancer treatments, a targeting
smart anti-cancer agent, FSB/38 micelles, which
consisted of 80% SD and 20% targeting copolymers
were successfully designed and produced. It was
then confirmed that FSB/38 micelles are an ideal
anti-cancer drug through cytotoxicity experiments
and cellular uptake experiments. The in vitro
cytotoxicity results showed that FSB/38 micelles
achieved the best effectiveness in killing colon
Caco2 cells among the designed anti-cancer drugs.
Hence, these findings confirm the efficacy of
FSB/38 micelles as an effective chemotherapeutic
drug for colon cancer treatment.
ACKNOWLEDGEMENTS
This study was supported by the National Science
Council of the Republic of China (NSC 102–2320-
B-002–038-MY3). We appreciate the help from
National Taiwan University College of Medicine.
REFERENCES
Bala, V., Rao, S., Boyd, B. J. & Prestidge, C. A. 2013.
Prodrug And Nanomedicine Approaches For The
Delivery Of The Camptothecin Analogue Sn38. J
Control Release, 172, 48-61.
Chen, Y.-I., Peng, C.-L., Lee, P.-C., Tsai, M.-H., Lin, C.-
Y., Shih, Y.-H., Wei, M.-F., Luo, T.-Y. & Shieh, M.-J.
2015. Traceable Self-Assembly Of Laser-Triggered
Cyanine-Based Micelle For Synergistic Therapeutic
Effect. Advanced Healthcare Materials, 4, 892-902.
SN38 Concentration (μg/ml)
0 0.1 1 10 100
Cell viability (%)
0
20
40
60
80
100
120
NB/SN micelle
FSB/SN micelle
SN38
SB/SN micelle
SN38 Concentration (μg/ml)
00.010.1 1 10
Cell viability (%)
0
20
40
60
80
100
120
FSB/SN micelle PH=7.4
FSB/SN micelle PH=6.7
FSB/SN micelle PH=6
AB
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
220

Cuong, N.-V., Li, Y.-L. & Hsieh, M.-F. 2012. Targeted
Delivery Of Doxorubicin To Human Breast Cancers
By Folate-Decorated Star-Shaped Peg-Pcl Micelle.
Journal Of Materials Chemistry, 22, 1006-1020.
Estrella, V., Chen, T., Lloyd, M., Wojtkowiak, J.,
Cornnell, H. H., Ibrahim-Hashim, A., Bailey, K.,
Balagurunathan, Y., Rothberg, J. M., Sloane, B. F.,
Johnson, J., Gatenby, R. A. & Gillies, R. J. 2013.
Acidity Generated By The Tumor Microenvironment
Drives Local Invasion. Cancer Research, 73, 1524-
1535.
Huo, M., Yuan, J., Tao, L. & Wei, Y. 2014. Redox-
Responsive Polymers For Drug Delivery: From
Molecular Design To Applications. Polymer
Chemistry, 5, 1519-1528.
Joralemon, M. J., Mcrae, S. & Emrick, T. 2010. Pegylated
Polymers For Medicine: From Conjugation To Self-
Assembled Systems. Chemical Communications, 46,
1377-1393.
Kawato, Y., Aonuma, M., Hirota, Y., Kuga, H. & Sato, K.
1991. Intracellular Roles Of Sn-38, A Metabolite Of
The Camptothecin Derivative Cpt-11, In The
Antitumor Effect Of Cpt-11. Cancer Research, 51,
4187-4191.
Khatik, R., Dwivedi, P., Junnuthula, V. R., Sharma, K.,
Chuttani, K., Mishra, A. K. & Dwivedi, A. K. 2015.
Potential In Vitro And In Vivo Colon Specific
Anticancer Activity In A Hct-116 Xenograft Nude
Mice Model: Targeted Delivery Using Enteric Coated
Folate Modified Nanoparticles. Rsc Advances, 5,
16507-16520.
Lai, T. C., Cho, H. & Kwon, G. S. 2014. Reversibly Core
Cross-Linked Polymeric Micelles With Ph- And
Reduction-Sensitivities: Effects Of Cross-Linking
Degree On Particle Stability, Drug Release Kinetics,
And Anti-Tumor Efficacy. Polymer Chemistry, 5,
1650-1661.
Peng, C.-L., Shih, Y.-H., Lee, P.-C., Hsieh, T. M.-H., Luo,
T.-Y. & Shieh, M.-J. 2011a. Multimodal Image-
Guided Photothermal Therapy Mediated By 188re-
Labeled Micelles Containing A Cyanine-Type
Photosensitizer. Acs Nano, 5, 5594-5607.
Peng, C. L., Shieh, M. J., Tsai, M. H., Chang, C. C. & Lai,
P. S. 2008. Self-Assembled Star-Shaped Chlorin-Core
Poly(Epsilon-Caprolactone)-Poly(Ethylene Glycol)
Diblock Copolymer Micelles For Dual Chemo-
Photodynamic Therapies. Biomaterials, 29, 3599-608.
Peng, C. L., Tsai, H. M., Yang, S. J., Luo, T. Y., Lin, C.
F., Lin, W. J. & Shieh, M. J. 2011b. Development Of
Thermosensitive Poly(N-Isopropylacrylamide-Co-((2-
Dimethylamino) Ethyl Methacrylate))-Based
Nanoparticles For Controlled Drug Release.
Nanotechnology,
22, 265608.
Peng, C. L., Yang, L. Y., Luo, T. Y., Lai, P. S., Yang, S.
J., Lin, W. J. & Shieh, M. J. 2010. Development Of Ph
Sensitive 2-(Diisopropylamino)Ethyl Methacrylate
Based Nanoparticles For Photodynamic Therapy.
Nanotechnology, 21, 155103.
Sinn Aw, M., Kurian, M. & Losic, D. 2014. Non-Eroding
Drug-Releasing Implants With Ordered Nanoporous
And Nanotubular Structures: Concepts For Controlling
Drug Release. Biomaterials Science, 2, 10-34.
Vaupel, P., Kallinowski, F. & Okunieff, P. 1989. Blood
Flow, Oxygen And Nutrient Supply, And Metabolic
Microenvironment Of Human Tumors: A Review.
Cancer Research, 49, 6449-6465.
Xing, Q., Li, N., Jiao, Y., Chen, D., Xu, J., Xu, Q. & Lu, J.
2015. Near-Infrared Light-Controlled Drug Release
And Cancer Therapy With Polymer-Caged
Upconversion Nanoparticles. Rsc Advances, 5, 5269-
5276.
Xu, S., Olenyuk, B. Z., Okamoto, C. T. & Hamm-Alvarez,
S. F. 2013. Targeting Receptor-Mediated Endocytotic
Pathways With Nanoparticles: Rationale And
Advances. Adv Drug Deliv Rev, 65, 121-38.
A Targeting Self-breakable Agent for Increased Efficacy of Chemotherapeutic Drugs against Caco2 Cells
221
