
Supporting Novice Prehospital Transcranial Ultrasound Scanning
for Brain Haemorrhage
Leila Eadie
1
, Luke Regan
2
, Ashish MacAden
2
and Philip Wilson
1
1
Centre for Rural Health, University of Aberdeen, Centre for Health Science, Old Perth Road, Inverness, U.K.
2
NHS Highland, Raigmore Hospital, Old Perth Road, Inverness, U.K.
Keywords: Ultrasound, Prehospital, Remote, Support, Transcranial, 3-Dimensional, Haemorrhage.
Abstract: Traumatic brain injury is a significant problem due to difficulties in early diagnosis in the field. Computed
tomography is the gold standard for detecting brain haemorrhage, but scanners are bulky and expensive. A
cheap, portable scanner such as transcranial ultrasound (TCUS) could allow early triage and intervention.
Transmitting images to remote experts for diagnosis means TCUS could be used by any minimally trained
person in the field. We propose a virtual 3-dimensional model of the head which shows which areas of the
brain have been imaged already, where the probe currently is, and where still needs to be covered in order to
generate a complete scan. Using sensors to measure the position and rotation of the TCUS transducer, we
can link this to the 3D model of the head and visually display which areas have been imaged. The images
can be analysed and composited to form a personalised 3D scan with maximal coverage of the brain, which
can be transmitted for diagnostic review, reducing data loss compared with streaming ongoing images.
Initial testing of the software has been performed in healthy volunteers and further testing is planned in
patients with brain haemorrhage.
1 INTRODUCTION
Traumatic brain injury (TBI) is a significant
problem, with challenges in diagnosis, especially
early diagnosis in the field. Closed head injuries are
of particular concern and far outnumber the
penetrative head injuries on which official statistics
are based. Currently, there are no well-accepted
diagnostic tests for use in standard medical practice
to diagnose TBI (Centers for Disease Control and
Prevention [CDC] et al., 2013). Ideally, brain
imaging should be performed as soon as possible
because posttraumatic bleeding within the skull is
associated with worse prognosis and can be life
threatening (CDC et al., 2013). Prompt diagnosis
and improved prehospital care can mean that
secondary (non-immediate) brain injury can be
prevented or limited by good early care (maintaining
blood and oxygen flow to the brain, controlling
blood pressure and haemorrhage and potentially
drilling of surgical burr holes), training and
organisation of trauma services, potentially leading
to significant reductions in both mortality and long-
term disability (Gentleman, 2008; CDC et al., 2013).
Computed tomography (CT) scanning is the gold
standard for detecting haemorrhage in the brain, but
is not feasible in the field: the scanners are heavy,
bulky, expensive and currently not developed in any
ruggedized form. A cheap, portable scanner for
bleeding in the brain (plus other conditions such as
skull fractures, indicators of intracranial pressure,
etc.) could alert medics to problems and allow early
triage and intervention. We believe transcranial
ultrasound (TCUS) has potential in this area and, if
used with a communications system to transmit the
images to a remote expert for diagnosis, could be
used to assess the injured by any minimally trained
person in the field. These scanners can fit into cases
only a little larger than a laptop, and can therefore be
used in ambulances and other prehospital situations.
Ultrasound is a useful tool for many diagnostic
purposes in trauma and beyond, and it is hoped that
this work will add to its utility rather than requiring
a different tool for head injuries.
Currently ultrasound is not routinely used
clinically for identifying brain structures and
abnormalities, although transcranial Doppler,
measuring blood flow velocity within cerebral
arteries, is a more commonly performed scan
(Sarkar et al., 2007). However, following previous
work by our group – the Satellite Ultrasound for
118
Eadie, L., Regan, L., MacAden, A. and Wilson, P.
Supporting Novice Prehospital Transcranial Ultrasound Scanning for Brain Haemorrhage.
DOI: 10.5220/0005789901180123
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 2: BIOIMAGING, pages 118-123
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Rural Stroke project (Mort et al., 2015) – we believe
that TCUS could provide useful prehospital
information when imaging brains looking for
haemorrhage. In this project, prehospital ultrasound
images were recorded by novice users and streamed
live using mobile or satellite networks from an
ambulance in remote and rural areas of the Scottish
Highlands to hospital-based experts for diagnosis.
The quality of the real-time streamed images was
rated and found to be good enough for diagnostic
purposes in ~93% of cases, although connectivity
was variable. This preliminary work identified that
novice scanners could, after brief training, record
images of the brain’s midline, located using the third
ventricle. The next logical step was to focus on
facilitating the TCUS scanning by novices to ensure
that the appropriate information is captured and
easily and efficiently transmitted.
If TCUS is to be used to provide information
about bleeding in the brain (or its absence), then it is
important to ensure that as much of the brain has
been scanned as possible so the accuracy of the
diagnosis can be quantified. Novice scanners in
particular may find this difficult, without the expert
knowledge to interpret the images and direct their
scanning. It would be useful to provide them with a
guide showing what they have scanned and what has
not yet been imaged.
The current research is developing a software
package to assist the operator in achieving
comprehensive ultrasound scanning of the head. We
aim to make TCUS scanning simpler for non-
medically trained users by providing them with a
visual 3D model of the head which clearly shows
which areas of the brain have been imaged already,
where the probe currently is, and where still needs to
be covered in order to generate a maximal scan –
something that is important for diagnosing and
treating brain injury. By measuring the position and
rotation of the ultrasound transducer using
movement sensors, we can determine where the
probe is pointing at all times during the scanning
session. Linking this to a 3D model of the head
allows us to determine which areas have been
imaged and which have not. The images recorded
will then be composited to form a complete 3D scan
of the subject’s head, using image processing
techniques to locate the skull in order to fit the
model to the individual’s specific head size and
shape. This means a personalised 3D TCUS scan
with maximum coverage of the brain is created,
offering the ability to view the brain images from
any point on the head, as if the user possessed a
virtual probe that can be placed anywhere on the 3D
model. This 3D scan is also a single file that can be
transmitted for diagnostic review with less chance of
data loss than streaming images from an ongoing
scan.
This position paper describes the initial tests in
healthy volunteers, and discusses plans for further
development and testing.
2 METHODS
The software was written using MATLAB R2015a
(MathWorks, Massachusetts USA) and produces a
real-time display of where the TCUS transducer is
currently pointing and has already scanned; plus
offline image analysis to locate the skull in the
recorded TCUS images and use this to deform a
standard 3D skull mesh. The scan model is displayed
and interacted with on a laptop, but in future work
could potentially be integrated into manufacturers’
ultrasound software.
A Philips CX50 portable ultrasound machine
(Philips Healthcare, Amsterdam, Netherlands) was
used in the testing, with an inertial measurement unit
sensor (3-Space Sensor, YEI Technology, Ohio
USA) attached to the transducer throughout
scanning. One temporary experimental set-up is
shown in Figure 1; ideally the sensor chip would be
integrated into the probe for easier handling. This
sensor records quaternion measurements which are
then used by the new software to calculate the plane
of the scan, which is then displayed within the skull
mesh. There are currently no dedicated TCUS
transducers available, so volunteers used a cardiac
probe with an appropriately small footprint, which
can be used at lower frequencies (1-5 MHz) to
penetrate through the skull and image to the opposite
side of the cranial vault; software settings of the
Figure 1: The inertial measurement unit sensor and the
ultrasound probe.
Supporting Novice Prehospital Transcranial Ultrasound Scanning for Brain Haemorrhage
119
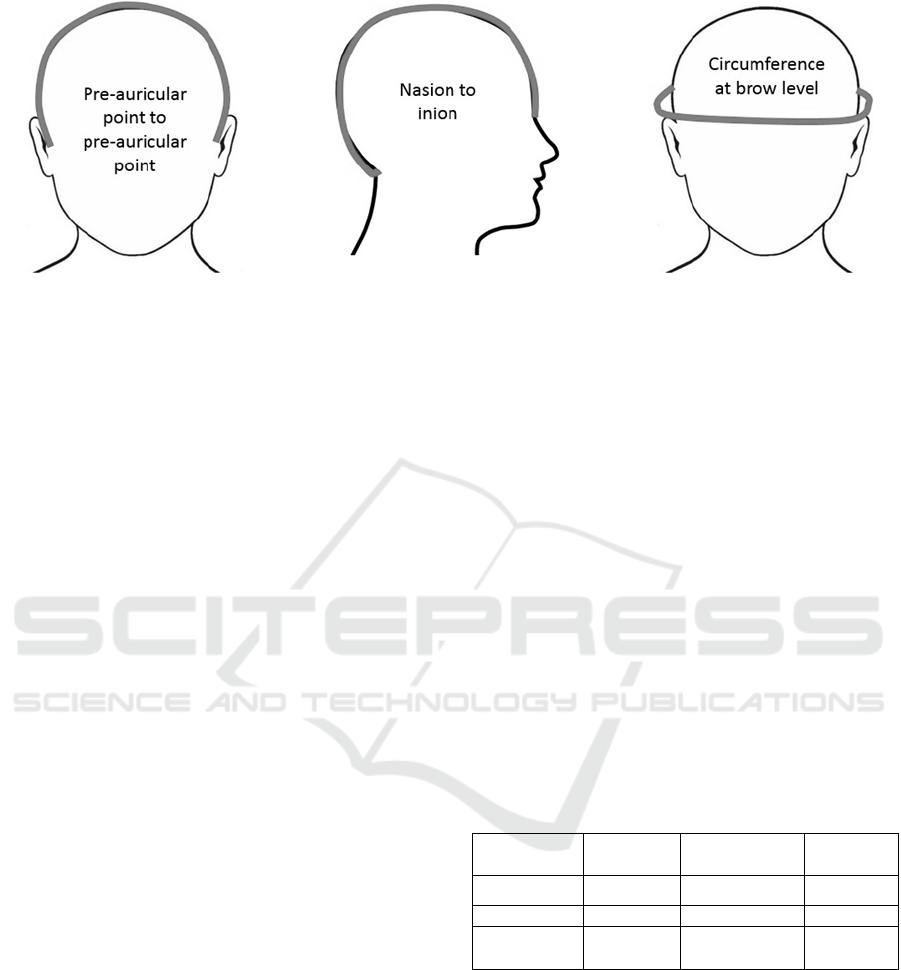
Figure 2: The head measurements made in the healthy volunteer study.
ultrasound machine were also optimised as much as
possible for head scanning.
The TCUS images were recorded as video clips
to capture both the images and any changes in depth,
power and sensitivity required to image the various
depths of brain for each volunteer. The images
recorded will be composited to form a complete 3D
scan of the subject’s head, using image processing
techniques (segmentation) to locate the skull in each
image in order to deform and fit the virtual 3D mesh
model to the individual’s specific head size. This
creates a personalised 3D TCUS scan.
Initial testing of the software was performed in
healthy volunteers to gather data about the scanning
process and visibility of brain structures on
ultrasound, plus transducer position data to calculate
the brain coverage of scans. Previous studies have
shown that between 5–20% of subjects have acoustic
windows offering reduced TCUS penetration, which
makes imaging more difficult or impossible (Seidel
et al., 1993; Sarkar et al., 2007).
Tests were done under laboratory conditions.
The dimensions of each volunteer’s head were
measured using standard neurophysiological
landmarks (nasion, inion, preauricular points; see
Figure 2). Structural imaging involved scanning
through the left transtemporal acoustic window (an
area of often thinner bone used to allow greater
penetration of the ultrasound waves) to the opposite
side of the head with the transducer in the vertical
plane, then determining the lateral and vertical range
of imaging achievable through this window.
Visualisation of landmarks such as the skull base,
brainstem, sphenoid bone and choroid plexus was
attempted. These steps were repeated in the
horizontal transducer plane, and from the opposite
transtemporal acoustic window. Imaging then
focussed on the midline of the brain, represented by
the third ventricle. The distance from skull to third
ventricle was measured from both transtemporal
windows. Volunteers then scanned a member of the
study team with the help of the new head scanning
software, after which they were given a short
questionnaire about their experience using the
program. Feedback was used to improve the
program’s usability and features.
3 RESULTS
The initial testing recruited 12 healthy volunteers: 9
female, 3 male, none of whom had used ultrasound
before. Table 1 shows the average head
measurements recorded in the study, grouped by
gender. This shows that there are differences in head
size that support the use of a personalised head
model rather than simply using a standard ‘average’
mesh model.
Table 1: The mean head measurements ± standard
deviation (in cm) recorded in the healthy volunteer study.
Subject type Nasion to
inion
Pre-auricular
point to point
Circum-
ference
Average
37.6 ± 1.7 35.7 ± 2.5 57.8 ± 1.6
Average male
38.3 ± 0.5 37.3 ± 2.6 58.7 ± 0.9
Average
female
37.3 ± 1.9 35.1 ± 2.1 57.6 ± 1.7
Two of the volunteers had transtemporal acoustic
windows that allowed more limited views of the
brain than others. This proportion is similar to what
has been reported in the literature. Imaging was still
possible, but of reduced clarity.
All twelve volunteers used the new software to
help them scan a team member’s head. Figure 3
shows a screenshot of the software from such a scan,
illustrating the cumulative coverage of the head
scans. The average scan time was 8 minutes, 47
seconds (range: 6 minutes, 1 second to 13 minutes,
52 seconds). The computational time of the program
BIOIMAGING 2016 - 3rd International Conference on Bioimaging
120
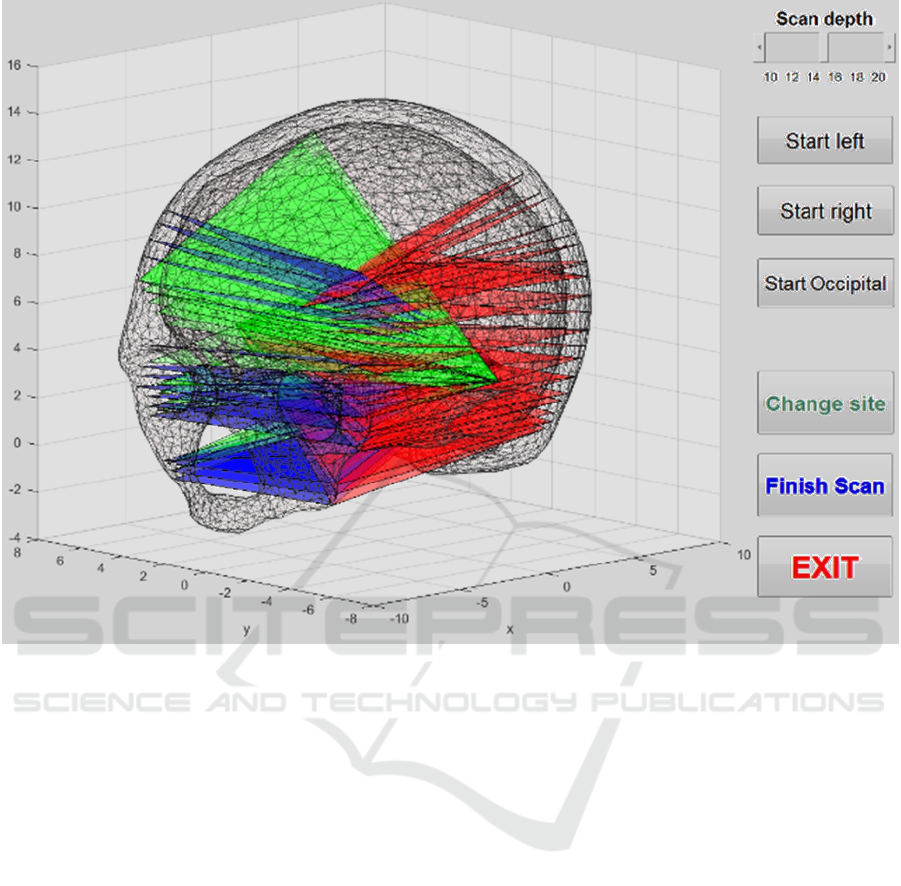
Figure 3: A screenshot of the 3D head model display program showing where scans have been performed from the different
acoustic windows (red for the patient’s right temporal window, green for their left temporal window and blue for the
occipital window).
was not an issue: it averaged approximately 0.3
seconds, with a maximum of 1 second seen during
the testing.
All volunteers said they felt they could use the
3D head model program, with 92% saying it was
easy to use. Some commented that they found using
the probe difficult initially, but this was because they
had no prior experience with ultrasound: more than
half (58%) said they thought they needed more
instructions or help, such as knowing exactly where
and how to place the probe (e.g., finding the
optimum window, which is actually done by
exploring the approximate area and locating the spot
that produces the best images; it is difficult to give
more than a general guide to location); and being
reminded to make sure the probe didn’t slide away
from the window due to the ultrasound gel used.
All of the volunteers reported that they thought
the 3D model accurately represented the direction of
the probe and that the model updated quickly
enough as they moved. All also thought the model
correctly showed areas they had scanned. Only 36%
said they were able to scan the areas the model
showed were unscanned. This is most likely because
there will be areas, such as the very top of the head,
where the probe cannot scan because it loses contact
with the head when tilted to the necessary angle. The
volunteers did not scan from the occipital window
(due to the poor images seen from this site when it
was attempted), which might have allowed them to
scan some areas otherwise unreachable.
Finally, all of the volunteers reported they
thought the program included all the functions they
might expect to find, given its aims, and all also
were satisfied that the program achieved these aims.
The volunteers were asked to rate the program as
a whole on a scale of 1 (useless) to 5 (useful), and
the mean rating was 4.3, the median was 4 (25
th
–
75
th
percentiles: 4–4.5).
Suggestions from the volunteers as to how the
program could be improved included:
• Providing a more in-depth tutorial on ultrasound,
the machine and the probe, which is the sort of
training that would be provided to end users.
Supporting Novice Prehospital Transcranial Ultrasound Scanning for Brain Haemorrhage
121

• Having a probe designed specifically for head
imaging, with a better shape for reaching all areas;
and incorporating the 3D-model into the ultrasound
screen so it is easier to see both at once. Addressing
both of these points could involve working with
ultrasound manufacturers for further development.
• Indicating the places on the model where they are
unlikely to be able to image.
• Showing a different coloured plane to indicate
where the probe is currently imaging, which has
since been implemented.
• Highlighting any abnormalities imaged, which
would involve computer-aided diagnosis analysis,
discussed in the Future Work section of this paper.
• Automatically detecting when the probe has been
removed from the patient’s head (currently this is
done manually by pressing a button). This is a point
for future development, but a partial solution has
been implemented, detecting when the probe is held
upright, a position in which it would not usually be
during usual TCUS scanning.
4 FUTURE WORK
The analysis of the ultrasound data is ongoing,
looking at forming 3D scans from the 2D images
collected, and calculating the variation between the
scans of the research team member that were taken
by different users.
The patient scanning study is currently starting
and involves hospital-based testing of the software
package in up to 10 patients with bleeding in the
brain. Images of the bleeding are collected for use in
two ways: to test the scanning support software in a
controlled clinical environment, and to compile a set
of TCUS scans featuring haemorrhage, to help
investigate the appearance of bleeding in the brain
on ultrasound. This appearance will change as the
time from the bleeding event increases and the blood
clots, so it is important to image several different
patients with brain haemorrhage (ideally as a result
of different causes), to capture a range of
haemorrhage images. These will also be used to
explore the potential for automated computer-
assisted diagnosis to support TCUS assessment of
patients with brain injury.
Details of the diagnosis and timing of the brain
haemorrhage will be recorded, as will CT images
that form part of the patient’s usual care and
diagnosis, for comparison. Scanning will take place
at the patient’s bedside and involve the same
structural scanning as in the healthy volunteer study,
plus the area of the haemorrhage will be
comprehensively imaged. The expert ultrasound
operators will provide feedback via a short
questionnaire; they will also be asked to assess the
resulting 3D image models for utility and quality at a
later time point.
5 CONCLUSIONS
The software described in this paper is specifically
designed to support non-medically trained TCUS
users in taking diagnostically useful images, so that
no expertise in interpreting ultrasound is required.
Brief training on the use of the ultrasound machine
and 3D imaging program is all that is needed. It has
been initially tested in healthy volunteers, with
further testing planned in patients with brain
bleeding.
This study recruited volunteers for whom it was
their first time using ultrasound, and they took a
little time to become comfortable with holding the
probe and finding the optimum acoustic windows
through which to image. The average scan time of
almost 9 minutes would no doubt improve with
practice, experience and confidence. The
computational time of the program did not produce
any appreciable lag and the feedback provides
essentially updates in ‘real time’. The scan model
used in this work was displayed and interacted with
on a laptop, but in future work could potentially be
integrated into manufacturers’ ultrasound software,
so that users do not have to view two different
screens.
The volunteers reported that the support system
fulfilled its aims and appeared to be working
correctly. They also made suggestions for
improvements, some of which have already been
implemented and will be ready for testing in further
trials. This study was the first feasibility test and
highlighted some problems with the imaging system,
such as the existence of areas of the brain that are
extremely difficult to scan with the currently
available hardware. Using additional acoustic
windows could help provide solutions to this
problem, as could development of a TCUS-specific
probe.
TBI is a significant problem, especially for the
military, and can lead to neuropsychiatric and
neurological sequelae. The benefits of a field-based
lay-user TCUS system are applicable to both
military and civilian situations (e.g., prehospital,
ambulance-based diagnosis for head injury and
stroke). It builds on existing (currently experimental)
ultrasound transmission technology to improve its
BIOIMAGING 2016 - 3rd International Conference on Bioimaging
122

usability and efficiency for use by non-expert
medics. The proposed system will make it easy for
any minimally trained personnel to collect
diagnostically relevant head images, which can then
be transmitted in a single package to a remote site
for interpretation. In this way, early diagnosis of
brain injury in the field – specifically looking for
haemorrhage in closed traumatic head injury - can
be improved without requiring major training for
novice users, because, thanks to modern
communication technologies, the images can be
transmitted and diagnosis performed by experts at a
remote site. The proposed system will also decrease
the effects of unstable transmission and packet loss
in sending the images to experts, by compositing the
data into a single file to be transmitted rather than
streaming the ultrasound video, where frames will
frequently be lost.
The impact of earlier diagnosis of TBI using
such a system as described here could be potentially
huge (preventing/minimising sequelae, long term
health effects of early/any treatment). It does rely on
TCUS being able to reliably detect haemorrhage in
the brain, but previous studies have shown there is a
strong possibility that the sensitivity will be of a
useful level (e.g., Mäurer et al., 1998). These studies
were performed some time ago so, with the benefit
of today’s improved ultrasound technology and
ongoing transducer optimisation, we are optimistic
that TCUS will prove worthwhile and useful for this
situation. We are planning concurrent validity
studies with TCUS and CT in patients with stroke in
order to provide updated evidence that modern US
systems can be used to reliably detect haemorrhage.
Although TCUS may be less sensitive than CT for
detecting haemorrhage, its portability and low cost
make it an attractive technology for battlefield and
transit use. Ruggedised systems are already available
for use in the field, and are used by air ambulance
services around the world.
We believe TCUS has potential and, if used with
a communications system to transmit the images to a
remote expert for diagnosis, could be used to assess
the injured by any minimally trained person in the
field.
ACKNOWLEDGEMENTS
This project is funded by the UK Ministry of
Defence, Defence Science and Technology
Laboratory. The ultrasound machine used was
loaned to the Centre for Rural Health by Philips
Healthcare.
REFERENCES
Centers for Disease Control and Prevention, the National
Institutes of Health, the Department of Defense, and
the Department of Veterans Affairs Leadership Panel.
2013. Report to Congress on traumatic brain injury in
the United States: Understanding the public health
problem among current and former military
personnel.
Cronk, T.M. 2012. Military Leads in Treating Traumatic
Brain Injury, Expert Says. Department of Defense
News, August 31 2012. Available from:
http://www.defense.gov/news/newsarticle.aspx?id=11
7724 [Accessed 05/11/2015].
Defense Centers of Excellence for Psychological Health
and Traumatic Brain Injury. 2010. Portable, field-
based devices for the early diagnosis of mild traumatic
brain injury. 2010
Gentleman, D. 2008. Synopsis of causation. Head injury.
Ministry of Defence. Available from:
http://www.veterans-uk.info/publications/
head_injury.pdf [Accessed 05/11/2015].
Mäurer, M., Shambal, S., Berg, D., Woydt, M., Hofmann,
E., Georgiadis, D., Lindner, A., Becker, G. 1998.
Differentiation between intracerebral hemorrhage and
ischemic stroke by transcranial color-coded duplex-
sonography. Stroke. Vol. 29, p.2563-2567.
Mort, A., Eadie, L., Regan, L., Macaden, A., Heaney, D.,
Mouley-Bouamrane, M., Rushworth, G., Wilson, P.
Combining transcranial ultrasound with intelligent
communications methods to enhance the remote
assessment and management of stroke patients –
Framework for a technology demonstrator. Health
Informatics Journal, in press.
Sarkar, S., Ghosh, S., Ghosh, S.K., Collier, A. 2007. Role
of transcranial Doppler ultrasonography in stroke.
Postgraduate Medical Journal. Vol. 83, p.683-689.
Seidel, G., Kaps, M., Dorndorf, W. 1993. Transcranial
color-coded duplex sonography of intracerebral
hematomas in adults. Stroke. Vol. 24, p.1519-1527.
Supporting Novice Prehospital Transcranial Ultrasound Scanning for Brain Haemorrhage
123
