
Determination of Ascorbic Acid Level in Orange Juice using an
Open-source Poteniostat & Screen Printed Electrodes
Ahmad M. Ali, Iman Morsi and Maha Sharkas
Arab Academy for Science & Technology, Department of Electronics & Communication Engineering,
Abu Qear, Alexandria, Egypt
Keywords: Ascorbic Acid, Poteniostat, Cyclic Voltammetry, Orange Juice.
Abstract: In this research we describe the use of cyclic voltammetry concept in order to determine the level of
Ascorbic Acid in orange juice. The proposed method consists of an open-source poteniostat and screen
printed electrodes. The Current result from the chemical reaction is proportional to the concentration of the
Ascorbic Acid. This method was applied to different commercial samples of orange juice and the results
were used to determine which one has the most preservation of Ascorbic Acid.
1 INTRODUCTION
Ascorbic Acid (Vitamin C) is a water-soluble
vitamin that is highly needed to the human body.
The human body needs Ascorbic Acid (AA) to
produce collagen to make connective tissue. (AA) is
also important the body to absorb iron, heal wounds,
build red blood cells and to fight infections. Since
orange is a great source of (AA) our study is
conducted on different samples of orange juice.
There are several factors that affect the amount
of (AA) in orange juice such as heat, light, exposure
to oxygen and how the juice is made during the
manufacturing process. In this research we focused
the study on the effect of storage temperatures on,
both, fresh and packed orange juice.(Royston and
Angela, 2003).
According to the present life style, people prefer
depending on the packed juices as they are versatile,
accessible and just ready to drink. Consequently,
these products must be periodically checked to
ensure that they contain the right amount of each
nutritional element needed by the human body.
Considerately, the proposed method in this
research is to determine the level of (AA) in orange
juice which reflects the quality of the juice. By
exposing a variety of orange juice samples to
different storage temperatures and checking the
preservation of (AA).
There are some classical techniques for the
determination and assessment of (AA) such as:
titration with an oxidant solution, Chromatographic
methods and Fluorimetric methods. (A.M. Pisoschi
et al., 2008)
The need for another method arises from the
complexity of the previous methods, the expensive
components used and the use of large equipment. On
the other hand, the proposed method consists of a
hand-held poteniostat in the size of cell phone,
screen printed electrodes and a laptop to save the
readings. Fig.1
Figure 1: The poteniostat used in the research and the
screen printed electrodes.
2 BACKGROUND
Several researches have studied the determination of
ascorbic acid in orange juice or in other fruits. Each
study and research utilizes different equipment to
perform the cyclic voltammetry technique.
Fundamentally, Cyclic Voltammetry (CV) is a
58
Ali, A., Morsi, I. and Sharkas, M.
Determination of Ascorbic Acid Level in Orange Juice using an Open-source Poteniostat & Screen Printed Electrodes.
DOI: 10.5220/0005793700580063
In Proceedings of the 5th International Confererence on Sensor Networks (SENSORNETS 2016), pages 58-63
ISBN: 978-989-758-169-4
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

technique to study the behavior of electrochemical
reactions. In the CV, information about the samples
undergoing the test is obtained by measuring the
output anodic current as the input voltage varied (J.
Randles and Trans.Far, 1948).
To preform cyclic voltammetry on a certain
chemical element three electrodes are needed
(working, reference and counter) electrodes, a
poteniostat to vary the potential of the working
electrode and a recording device to measure the
output current.
It is worth mentioning that a research to
determine (AA) level in commercial fruit juices was
done using a potentiostat-galvanostat KSP,
laboratory made by SlawomirKalinowski and Pt disc
electrode as a working electrode, saturated calomel
electrode as the reference electrode and the counter
electrode was Pt strip. This setup proved its accuracy
in the determination and recovery of (AA) level in
fruit juices (Pisoschi et al., 2008).
The complication facing this method is that the
test must be done in a chemistry lab, as the
equipment used is static.
Another research was done on the determination
of (AA) level in orange juice using a poteniostat and
a lead pencil electrode. This method proved a
satisfactory determination of (AA) but with a
minimum concentration of 0.0326 mg/mL to
produce a linear determination curve (D. King et al.,
2010).
3 EXPERIMENTAL WORK
3.1 Samples
Three samples were taken from a freshly squeezed
orange juice and stored at 25 ºC, 18 ºC and 12 ºC for
the first experiment.
For the second experiment two samples from
commercial orange juice and one sample from a
freshly squeezed juice were stored at 18 ºC.
KCl solution with a concentration of 0.1 mole/L
was added to all samples as the KCL ions chemically
interact with (AA) ions and enhance the electric
conductivity of the (AA) molecules so that the anodic
current can be measured be the poteniostat.
3.2 Poteniostat and Screen Printed
Electrodes
3.2.1 Poteniostat
Poteniostats are amplifiers used to control the
voltage between working and reference electrodes.
A simple design of poteniostat is shown in Fig.2
(A.V. Gopinath and D.Russel, 2005).
Figure 2: Circuit Diagram of a simple poteniostat.
Several models and designs of poteniostats are
available in the market but due to their
uneconomical cost (range of thousands dollars) and
their huge equipment that is required to be fixed in a
certain place as they are not portable (A.V. Gopinath
and D.Russel, 2005).
The poteniostat used in this research is an open-
source device designed by a team in the
biochemistry department, University of California
Santa Barbra (Rowe AA et al., 2011).
The total cost of the used poteniostat is in the
range of (100$) which is inexpensive compared to
any model of a commercially available poteniostat.
Moreover, the poteniostat used is considered a
hand held device with a USB power supply. It can
be used anywhere due to its portability.
3.2.2 Screen Printed Electrodes
The elimination of bulky materials and instruments
is a major concern in the electrochemistry. The
printed electrodes offer high accuracy, low cost and
more portability.
A major advantage in using Screen Printed
Electrodes (SPE) is that it needs small sample
volume (~ µL) when compared to the traditional
solid electrodes (W. Wonsawat, 2014).
The SPE used in this research is manufactured by
Bio-Logic Science Instruments.
SPE consists of a Graphite working and counter
electrodes, and Ag/AgCl reference electrode with an
alumina substrate. Additionally, the SPE used allows
a low sample volume (25 µL-100 µL) which is a
great advantage in the on-site detection of the
sample under test.
Determination of Ascorbic Acid Level in Orange Juice using an Open-source Poteniostat & Screen Printed Electrodes
59
4 PROCEDURES
Two experiments were done to determine the level
of (AA) in orange juice. Both were done using the
cyclic voltammetry with the following parameters:
start voltage 200 mV, end voltage 900 mV, slope 50
mV/S, sample rate 5 mV/sample and number of
cycles equal to 1. The current has been evaluated at
550 mV because (AA) is highly chemically active at
this voltage so best current reading is taken at 550
mV (Rowe AA et al., 2011).
Both experiments were run for 7 days, with a
daily reading for each sample.
The SPE used was mechanically cleaned with
distilled water after each reading, also a potential is
applied on the SPE (-200 mV to 200 mV) after each
reading to ensure there is no interaction between the
sample under test and the electrode material (W.
Wonsawat,
2014).
For each reading 10 µL of KCl was added to 20
µL of orange juice sample and the mixture was
tested by the SPE and the poteniostat and the result
of each reading is stored in the PC.
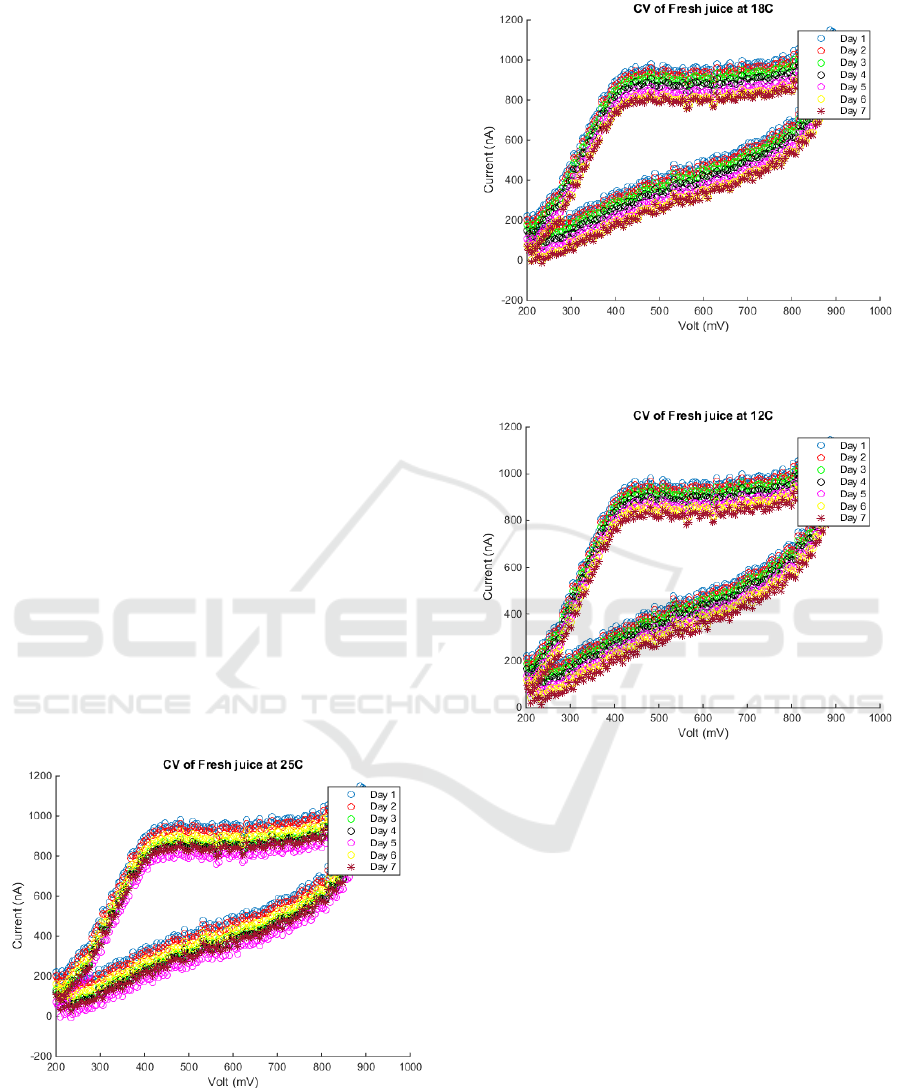
4.1 Experiment 1
In the first experiment three samples of freshly
squeezed orange juice were taken and stored at 25
ºC, 18 ºC and 12 ºC, in order to study the effect of
storage temperature on fresh orange juice. Fig.3
shows the cyclic voltammetry of (AA) at different
storing temperatures.
Figure 3a: Cyclic Voltammetry output of Fresh orange
juice stored at 25 ºC.
Figure 3b: Cyclic Voltammetry output of Fresh orange
juice stored at 18 ºC.
Figure 3c: Cyclic Voltammetry output of Fresh orange
juice stored at 12 ºC.
4.2 Experiment 2
In the second experiment three samples were used,
two of which were from commercial orange juice ,
one of them was a Natural Identical product and the
other was a Nectar product while the third sample
was a freshly squeezed orange juice, in order to study
which type will have the ability to preserve (AA) for
more time keeping the storage temperature (18 ºC.)
stable. Fig.4 shows the cyclic voltammetry for the
samples.
SENSORNETS 2016 - 5th International Conference on Sensor Networks
60
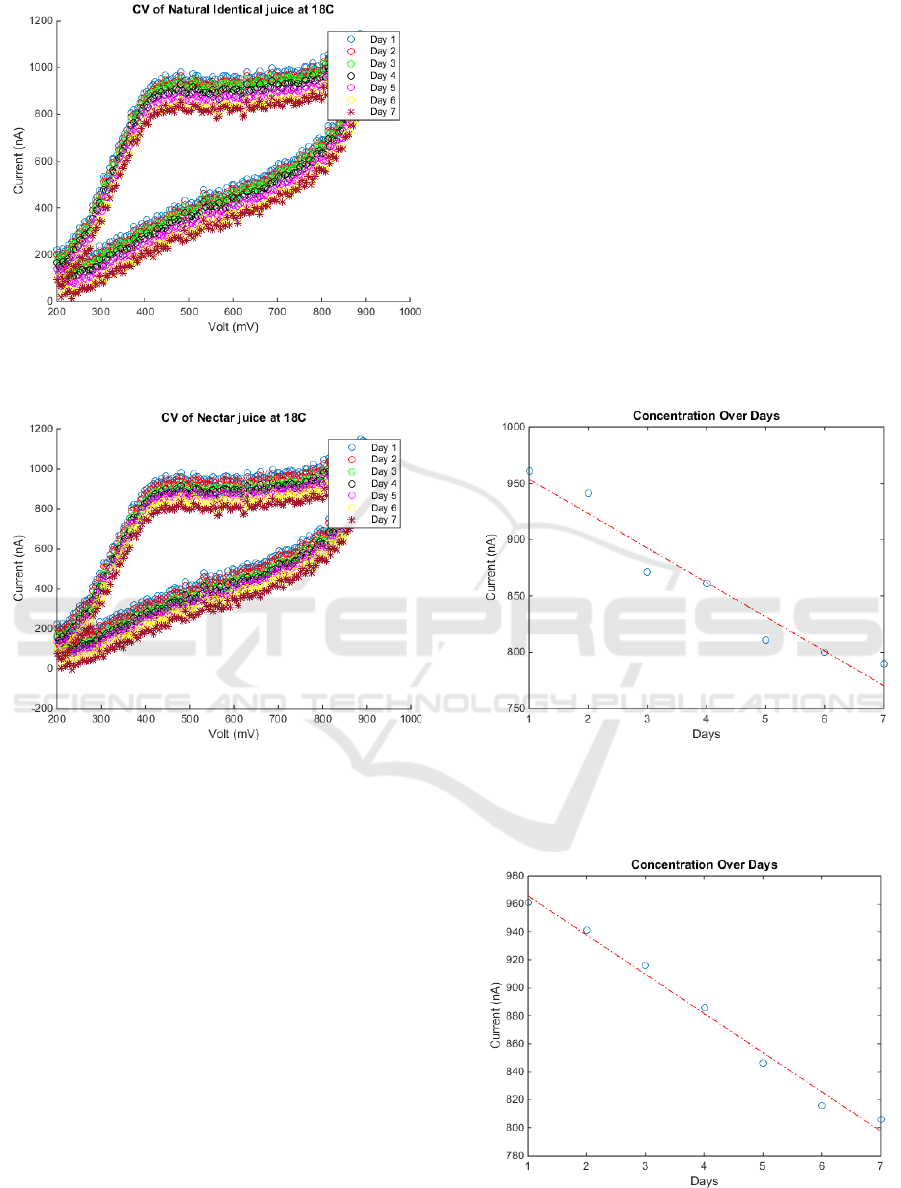
Figure 4.a: Cyclic Voltammetry output of Natural Identical
orange juice stored at 18 ºC.
Figure 4.b: Cyclic Voltammetry output of Nectar orange
juice stored at 18 ºC.
5 RESULTS AND DISCUSSION
According to Randles-Sevcik equation:
I
d
= 2.69.10
5
n
3/2
AD
1/2
v
1/2
c
(1)
Where c is the concentration, v is the voltage scan
rate, A is the electrode surface, D is the diffusion
coefficient, n is the number of electrons transferred
during the process and I
d
is the anodic current. (A.M.
Pisoschi et al., 2008)
It is obvious that the, concentration is directly
proportional to the anodic current. Accordingly, the
anodic current can be used as a mirror to the
concentration for the study of the (AA) behavior at
different storage temperatures.
5.1 Experiment 1 Results
Starting with the cyclic voltammetry curves, we can
deduce that for a freshly squeezed orange juice, the
lower the storage temprature, the more slower losing
the (AA).
The anodic current values at 550 mV were taken
to plot the relation between the anodic current and
measurments time because at 550 mV the ascorbic
acid ions are dominant over other ions in the orange
juice as shown in Fig5.
By performing a fitting curve to deduce the
equation that shows an approximate form of the
production time of the juice and hence control
techniques can be applied to accomplish different
qualities depending on the level of (AA). This
relationship is given by the following equations.
Figure 5.a: The anodic current at 550 mV at daily basis for
a fresh orange juice at 25 ºC.
y=-30.5357x+ 984.2857 (2)
Figure 5.b: The anodic current at 550 mV at daily basis for
a fresh orange juice at 18 ºC.
Determination of Ascorbic Acid Level in Orange Juice using an Open-source Poteniostat & Screen Printed Electrodes
61
y=-28.0357x+993.8571 (3)
Figure 5.c: The anodic current at 550 mV at daily basis for
a fresh orange juice at 12 ºC.
y=-22.0357x+988.1429
(4)
Equations (2, 3, 4) can be adopted to determine how
long has the juice been produced on top of checking
the expiry date that gives an indication for the
suitable use date.
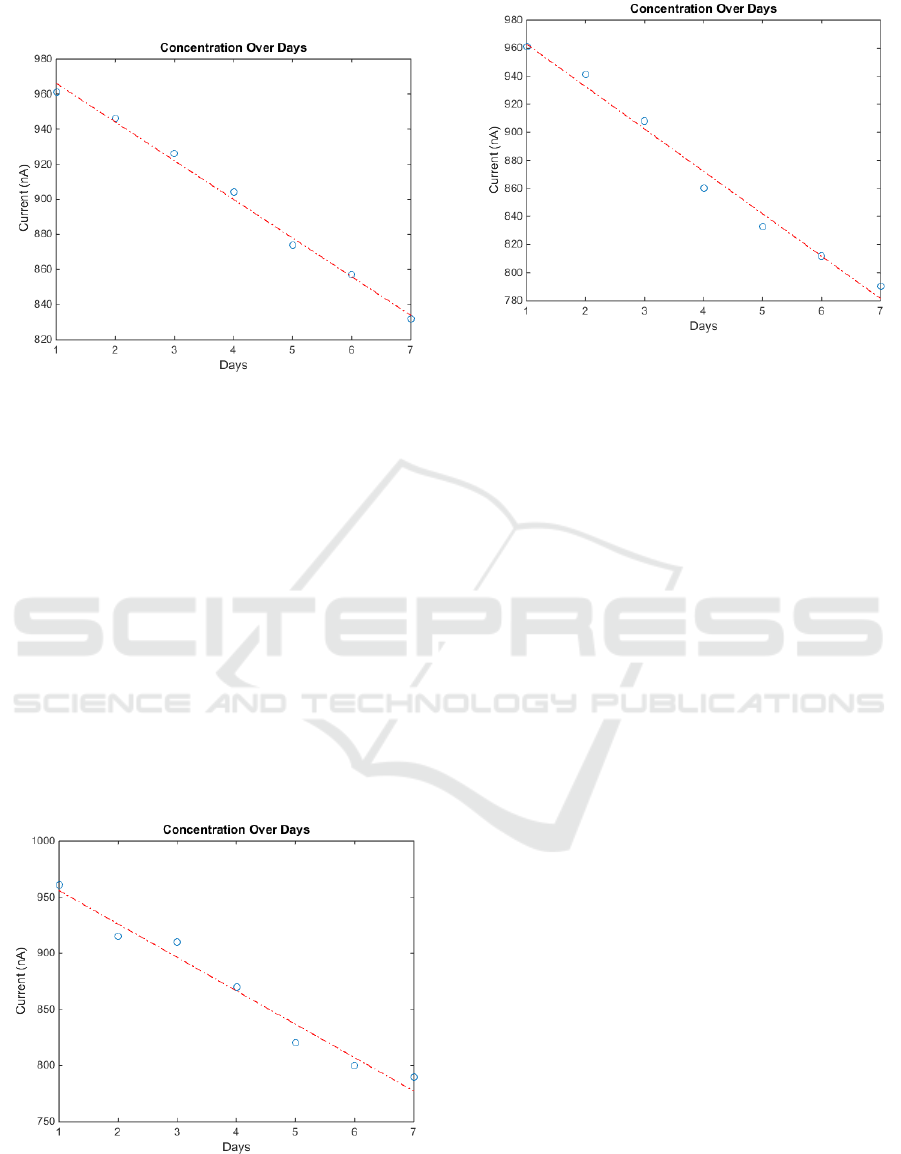
5.2 Experiment 2 Results
Arising from the cyclic voltammetry curves of the
three samples we can deduce that at the same storage
temperature, the freshly squeezed orange can
preserve (AA) for more time than the Natural
Identical and the Nectar. Fig.6 shows the degradation
rate of (AA) of the three juice samples at the same
temperature which is 18 ºC.
Figure 6a: The anodic current at 550 mV at daily basis for
a Natural Identical orange juice at 18 ºC.
y=-29.7500x+985.5714 (5)
Figure 6.b: The anodic current at 550 mV at daily basis for
a Nectar orange juice at 18 ºC.
y=-30.2143x+993.0000 (6)
We can also use equations (5, 6) to determine how
long has the juice been produced and check its
expiry date. Ergo, it can give an indication for the
best use date.
6 CONCLUSIONS
In this research we show how to employ the cyclic
voltammetry concept to determine the (AA) level in
orange juice. In this work we used an open-source
poteniostat which was chosen based upon its
inexpensiveness, portability and reliability for (AA)
measurements. Additionally, we used a low cost
screen printed electrodes for the complete process.
The results show that the freshly squeezed orange
juice at the lowest possible storage temperature can
preserve (AA) more than any type of orange juice at
the same temperature.
Wherefore, the deduced relation between the
anodic current and the time can be used to determine
the quality of the juice and an approximation to the
production and expiry dates.
REFERENCES
Royston, Angela (2003). Vitamins and Minerals for a
Healthy Body. Chicago, Illinois:Heinemann Library.
A.M. Pisoschi,A.F. Danet and S. Kalinowski “Ascorbic
Acid Determination in Commercial Fruit
JuiceSamples by Cyclic Voltammetry”Journal of
Automated Methods and Management in Chemistry,
vol.2008, no. 937651.
J. Randles, Trans. Far. Soc., 44 (1948) 327.
SENSORNETS 2016 - 5th International Conference on Sensor Networks
62

D. King, J. Friend and J. Karuki “Measuring Vitamin C
Content of CommercialOrange Juice Using a Pencil
Lead Electrode” Journal of Chemical Education,
vol.87 no.5, pp 507-508, May 2010.
A.V. Gopinath and D. Russell “An Inexpensive Field-
Portable Programmable Potentiostat” The chemical
Educator, vol.10, no.X, 2005.
Rowe AA, Bonham AJ, White RJ, Zimmer MP, Yadgar
RJ, Hobza TM, et al. (2011) CheapStat: An Open-
Source, “Do-It-Yourself” Potentiostat for Analytical
and Educational Applications. PLoS ONE 6(9):
e23783. doi:10.1371/journal.pone.0023783.
W. Wonsawat “Determination of Vitamin C (Ascorbic
Acid) in Orange Juices Product”, International
Journal of Biological, Food, Veterinary and
Agricultural Engineering, vol.8, no.6, 2014.
Determination of Ascorbic Acid Level in Orange Juice using an Open-source Poteniostat & Screen Printed Electrodes
63
