
Methylene Blue
A Trendy Photosensitizer in Medicine and in Solar-Energy Conversion Systems
Filipa Pires, Margarida Coelho, Paulo A. Ribeiro and Maria Raposo
Centre of Physics and Technological Research, CEFITEC, Department of Physics, Faculdade de Ciências e Tecnologias,
FCT, Universidade Nova de Lisboa, Caparica, Portugal
Keywords: Layer-by-layer, LbL, Methylene Blue, DNA, UV-Vis Spectroscopy, Solar Cell, Photodynamic Therapy.
Abstract: The photosensitizer methylene blue (MB) has been investigated as a deoxyribonucleic acid (DNA) intercalant
with the main objective of understanding the chemical/physical processes, which occur when adequate
wavelength light is impinging on DNA intercalated with MB. This understanding is crucial for the creation
of dynamic phototherapy procedures and for the development of new dye sensitized solar cells. During this
work we developed and optimized all the conditions to efficiently produce [MB/DNA] multi-layered films by
self-assembly. Our study revealed that pH strongly influences the growth of our multilayer films. Our UV
studies revealed that the UV radiation causes damage of DNA through opening of aromatic ring and by
breaking the DNA phosphate groups. The FT-IR studies on cast films with [MB/DNA] revealed that the
denaturation ratio decreases as the irradiation increases, meaning that MB is an intercalant of DNA chain.
This study paves the way to develop new dye sensitized solar cells which employ inexpensive materials and
take advantage of the intercalation process in order to adjust the intermolecular arrangement of the
donor/acceptor molecules to improve the device performance.
1 INTRODUCTION
Radiation exposure carries with it the risk of diseases
once it can damage crucial biomolecules such as
deoxyribonucleic acid (DNA), compromise the
imulogical and the nervous systems and contribute to
the development of cancer. (Teoule, 1987).
Several studies reported that ionizing radiation as
UV, X-rays, β and γ particles have enough energy to
excite or ionize the biomolecules. Interestingly, low-
energy species (secondary electrons) also induce
chemical and physical modifications in DNA such as
change of nucleobases, deletion of molecular groups
(phosphate and sugar) and both single and double
DNA strand formation.(Li et al., 2003) In healthy
physiological conditions, living systems have several
enzymatic DNA repair systems, which efficiently
remove this damage from DNA. If this damage is not
repaired in a cell, serious genetic changes such as
mutations occur, thus leading to cancer.
Photodynamic therapy destroys the target
malignant cells using a photosensitizer, a light
sensitive dye, which in the presence of oxygen,
activates and forms reactive oxygen species to induce
the cellular destruction. (Martinez and Chacon-
Garcia, 2005, Mansuri-Torshiza et al., 2001).
In 1992, Decher et al., introduced the layer-by-
layer technique: a revolutionary adsorption technique
consisting in the production of thin films by
immersing the film alternately in solutions of
oppositely charged materials with rinse steps in
between to remove any material unbound to the
surface.(Decher and Schmitt, 1992) LbL technique
requires small amounts of material offering a fine
control over the materials structure and is a cost-
effective, reproducible, robust and user-friendly
technique.
Lbl technique have been used in different areas
which integrate the health, electronics and
environment in order to develop smart nanostructured
devices, drug delivery systems, sensors and solar
cells.
In this paper, MB/DNA films were prepared using
the LbL technique and was assessed the influence of
several factors such as the pH, drying process and
immersion time in the loading of MB molecules. The
results showed that the pH value of MB solution has
a significant effect on the adsorption of the dye into
the film.
This study paves the way to develop new dye
Pires, F., Coelho, M., Ribeiro, P. and Raposo, M.
Methylene Blue - A Trendy Photosensitizer in Medicine and in Solar-Energy Conversion Systems.
DOI: 10.5220/0005843303790383
In Proceedings of the 4th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2016), pages 381-385
ISBN: 978-989-758-174-8
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
381
sensitized solar cells (Wang et al., 2010) which
employ inexpensive materials and take advantage of
the intercalation process in order to adjust the
intermolecular arrangement of the donor/acceptor
molecules to improve the performance of the device.
2 EXPERIMENTAL DETAILS
The LbL films were prepared from MB (MW= 373.90
g/mol) and DNA sodium salt from calf thymus,
obtained from Aldrich. The MB was dissolved in pure
water supplied by a Milli-Q system from Millipore
(resistivity of 18 MΩcm) to a concentration of 10 mM
and pH 7.The DNA aqueous solutions were 0.5
mg/mL concentration. The MB/DNA LbL films were
adsorbed onto quartz supports that had been
hydrophilized in a Piranha solution for 30 min.
Deposition comprised the following steps: (i)
immersion of the support in MB solution for 5 s; (ii)
washing the support plus MB layer with pure water;
(iii) immersion of the support plus MB layer into the
DNA solution for 60 s; and (iv) washing the substrate
MB/DNA bilayer with pure water. The number of
deposited bilayers is equal to the number of
repetitions of steps (i)–(iv).
Film growth was monitored by measuring the UV
visible using a Shimadzu UV-2101PC
spectrophotometer. The damage caused by UV
exposure was characterized using a FTIR
spectrophotometer Thermo Scientific Nicolet-model
530 (Waltham, MA, USA).
3 RESULTS AND DISCUSSION
3.1 [MB/DNA] Film Growth
The LbL technique is based on physical adsorption
processes resulting, mostly, from electrostatic
interactions but also the existence of van der Waals
forces, hydrogen bonding and hydrophobic forces.
(Oliveira Jr et al., 2001, Oliveira Jr et al., 2002).
Multilayers were deposited on quartz substrates
using the alternate dipping method into dilute
aqueous solutions of MB (pH=4) for 5 sec and DNA
(pH=6.8) for 60 sec. Contrary to what was expected,
the growth of the film is reduced, since the
absorbance values not suffered meaningful changes
as the increasing of the number of bilayers, since in
according to the literature, the amount adsorbed is
proportional to the number of bilayers.
Absorption bands at 260 nm (characteristic of
DNA and MB) and at 600 nm e 670 nm (characteristic
of MB) are clear evidences that some molecules were
adsorbed in the surface of substrates, possible
reflecting the non-ionic physical interactions. The
question we asked ourselves was: can the pH
influence the multilayers formation?
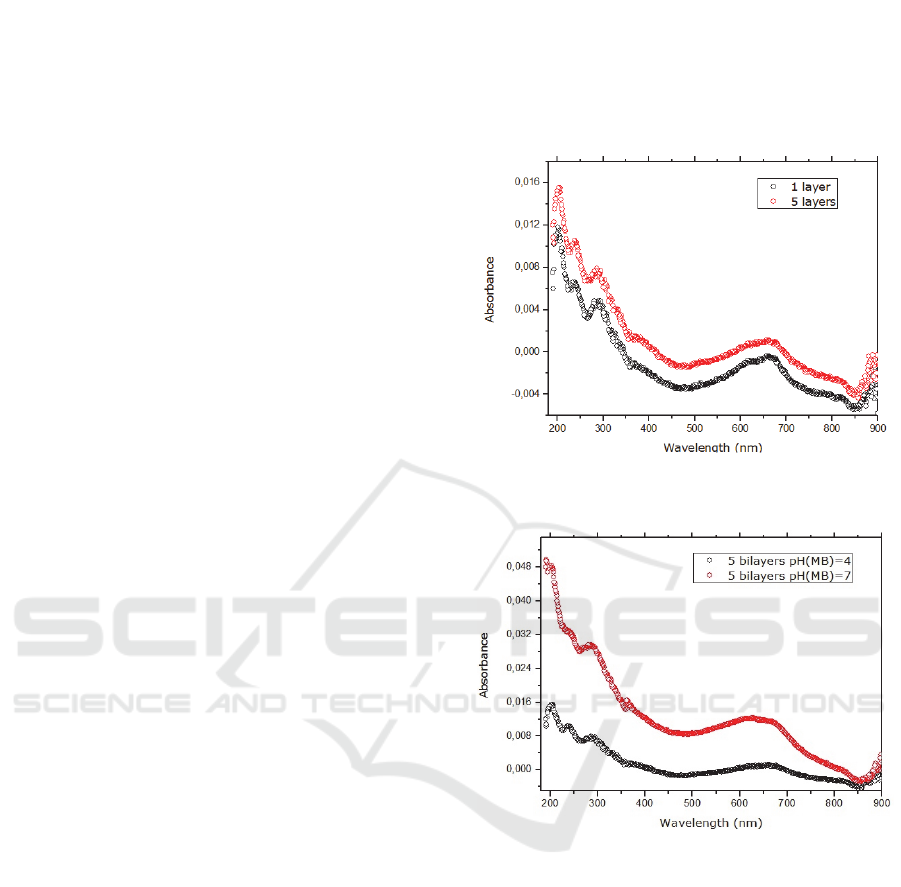
Figure 1: Absorption spectra of [MB/DNA]1 (black line)
and [MB/DNA]5(red line) films with AM at pH=4.
Figure 2: Absorbance spectra of [MB/DNA]5, a pH (MB) 4
(black line) and 7 (red line).
3.2 PH Affects MB Ionization and,
Consequently, the Growth of LbL
Films [MB/DNA]
Multilayers were deposited on quartz substrates using
dilute aqueous solutions of MB with a pH=7 instead
of a pH=4. Absorbance spectra of LbL films
[MB/DNA] with the same number of bilayers, at
different pH, are depicted in the Figure 2, showed that
the pH dramatically influences the growth of the
films. This can easily justified by the increase of the
degree of ionization of the molecules. In fact, at pH
7, the MB molecules are electrically charged which
contribute to the formation of the films due to the
ionic forces.(Impert et al., 2003) The absorption
AOMat 2016 - Special Session on Advanced Optical Materials
382
spectra of the LbL films, with the MB at pH = 7 shows
the characteristic absorption bands, (mentioned
before) of MB and DNA in the visible and ultraviolet.
Given that the assembly conditions were
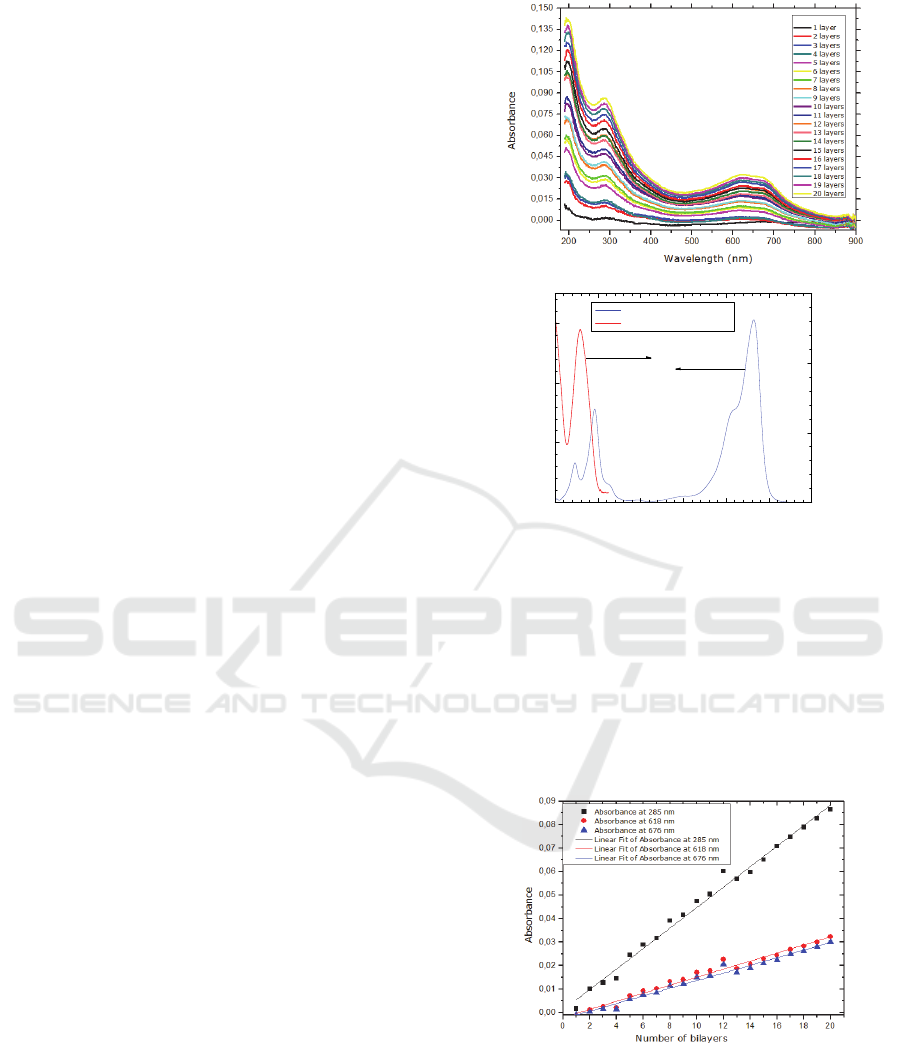
optimized, the quartz substrates were coated with 20
bilayers of MB/DNA. The absorption band at 618 nm
and 676 nm correspond to the dimeric and monomeric
form of MB, respectively. (Spencer and Sutter,
1979)In Figure 3, we can also see an absorption band
at 285 nm (characteristic of DNA and of MB) and
other at 292 nm which is characteristic of MB and,
according to literature, this assignment correspond to
→∗ transitions. It should be referred also that, for
these LbL films, the absorbance in the 600 to 700
range is lower than the absorbance in the 280 nm and
the absorbance has a nonzero value at 200 nm. As the
spectra of MB solutions the absorbance is higher in
the 600 to 700 nm than the absorbance at 280 nm and
the absborbance decreases to zero for 200 nm, see
figure 3b), one can conclude that both DNA and MB
molecules are incorporated in the films. These results
proved that both MB forms have the ability to link to
the DNA, allowing the formation of LbL films.
Compared to the absorption peak of aqueous MB
(at 664 nm), the visible maximum peak at 623 nm of
[MB/DNA]
20
film is blue-shifted by 41 nm.
According to Chao et al., phenothiazine dyes such as
MB, suffered a blue-shift absorption band because
they form H-aggregates via π-π stacking. (Gao et al.,
2008)
The absorbance at the peak (at 285 nm, 618 nm
and 676 nm) was used to monitor the buildup of
multilayers, as illustrated in Figure 4. One can see that
the data follow straight lines, indicating that the films
increase linearly with the number of bilayers,
meaning that each deposited bilayer contributes an
equal amount of deposited polymer.
The amount of MB adsorbed per bilayer was
calculated by taking the intensity of the 618 nm and
667 nm peak in the UV-Vis spectra for [MB/DNA]
20
.
From the absorbance intensity using the Beer-
Lambert law, we concluded that the MB adsorbed
layer per unit area was 96±5 µg/m
2
.
During this work, we assessed the influence of the
drying process as well the imersion time on the
morphology of the LbL films. The drying process
using a nitrogen stream (data not shown) seems to be
an important factor during the preparation of LbL
films since it promotes conformational changes of the
molecules through modifications of their
arrangement in space, which allows a greater
interaction between charges and facilitates the
bonding between the materials.
a)
b)
Figure 3: Absorption spectra of: a)[MB/DNA]20 LbL film.
b) aqueous solutions of MB and DNA.
The kinetic study of DNA imersion time (data not
shown), showed that imersion in DNA solution for 5
sec is enough to obtain a thin [MB/DNA] film with
all characteristic absorption bands of each material.
The imersion for long times can lead to a desorption
phenomena.
Figure 4: Maximum absorbance versus number of bilayers
of MB/DNA self-assembled films adsorbed on quartz
substrates.
200 300 400 500 600 700 800
0.0
0.2
0.4
0.6
MB (7.5M)
DNA (0.0075 mg/mL)
Wavelenght (nm)
Absorbance
0.00
0.05
0.10
0.15
Absorbance
Methylene Blue - A Trendy Photosensitizer in Medicine and in Solar-Energy Conversion Systems
383
3.3 FTIR Characterization of Films
The damage caused by UV radiation on DNA in the
presence of the intercalator MB, are characterized by
fourier transform infrared spectroscopy (FTIR). The
FT-IR results obtained to [MB/DNA]
100
film were
inconclusive since the adsorbed amount of MB and
DNA was not sufficient to obtain a spectra with
adequate resolution.
Cast films were prepared with mixture of DNA
and MB, and were irradiated for different time
intervals.
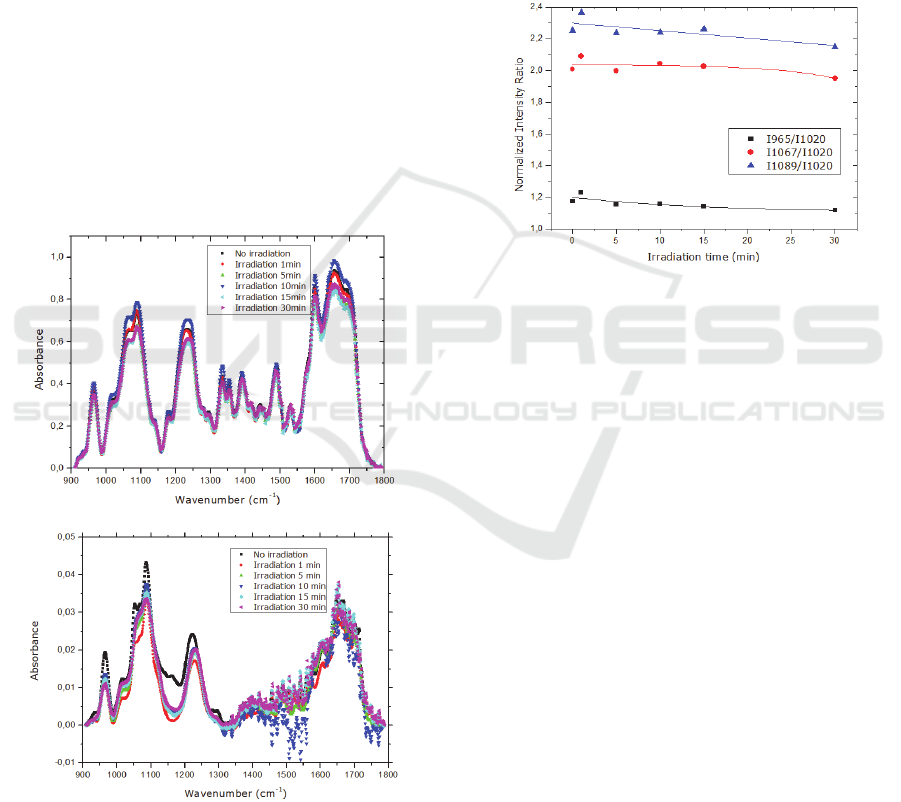
Several changes in the IR spectra of DNA and
mixture MB/DNA have seen observed after
irradiation, as illustrated in Figure 5a) and b). In order
to better analyse the infrared spectra changes, spectra
baselines were removed and the peaks which did not
change as a result of exposure to UV radiation were
identified. According to Gomes et al., the FT-IR band
detected near 1018 cm
-1
is very stable since it does
not suffer any change when exposed to a radiation
with 140nm. (Gomes et al., 2009)This peak is due to
a)
b)
Figure 5: Infrared absorbance spectra of: a) DNA cast
sample before and after irradiation with 254 nm UV light;
b)a mixture of MB/DNA cast sample before and after
irradiation with 254 nm UV light.
furanose vibrations and was used to normalize the
obtained data, dividing the other peaks areas by the
area of this peak, avoiding the possibility that the
small changes due to the measurement of the infrared
spectra in different regions of the sample are affecting
the observed peak areas decrease or increase.
The vibrations associated with C-O stretching of
nucleic acid sugar (1067 cm
-1
) and PO
2
- stretching
(1089 cm
-1
) decreased with the irradiation time.
According to Paulo et al.,
13-15
UV radiation causes
damage of DNA through opening of aromatic ring
and by breaking the DNA phosphate groups.
Figure 6: Normalized infrared intensity ratios at 965 cm-1
(CC stretch of backbone), 1067 cm-1 (CO stretch of the
furanose backbone), 1089 cm-1 (Symmetric PO2-
stretching of the backbone ) relative to peak area at 1020
cm-1 of a DNA cast sample irradiated for different periods
of time with 254 nm UV light. The solid lines are
guidelines.
As illustrated in Figure 6, as the irradiation time
increases the ratio of the peaks 1089 cm
-1
and 1067
cm
-1
decrease, meaning that radiation preferentially
affects the PO
2-
groups than sugars.
One of the goals of this work was understand if
the UV radiation leads to a DNA denaturation in the
MB/DNA film. The infrared intensity ratio at 1690
cm
-1
(C2=O2 strength of thymine single stranded or
double stranded and C6=O6 stretching of guanines),
was normalized relative to peak at 1652 cm
-1
(C2=O2
strength of cytosine single stranded or double
stranded and C4=O4 strength of thymine single
stranded or double stranded) of a DNA cast sample
irradiated for different periods of time with 254 nm
UV light. As the irradiation time increases the ratio
1690/1652 cm
-1
decreases, meaning that the presence
of MB induces an intercalation process which forces
the opening of the DNA chain.
AOMat 2016 - Special Session on Advanced Optical Materials
384

4 CONCLUSIONS
This work showed an efficient protocol to produce
[MB/DNA] multi-layered films by self-assembly.
The pH value should be 7, since at this pH the MB
molecules are electrically charged.
An MB adsorbed layer per unit area of 96±5
µg/m
2
was achived if the film is dried with nitrogen
at the end of each bilayer and immersed for 60 sec in
the DNA solution.
The UV studies revealed that the UV radiation
causes damage of DNA through opening of aromatic
ring and by breaking the DNA phosphate groups. Our
results also showed that UV radiation preferentially
affects the PO
2-
groups than sugars.
The FT-IR studies on cast films with [MB/DNA]
revealed that the denaturation ratio decreases as the
irradiation increases, meaning that MB is an
intercalant of DNA chain.
ACKNOWLEDGEMENTS
This work was supported by the Portuguese research
Grant UID/FIS/00068/2013 through FCT-MEC, the
"Plurianual" financial contribution of "Fundação para
a Ciência e Tecnologia" (Portugal). Filipa Pires
acknowledges the fellowship from RABBIT Doctoral
Programme (Portugal).
REFERENCES
Decher, G., Schmitt, J. 1992. Fine-tuning of the film
thickness of ultrathin multilayer films composed of
consecutively alternating layers of anionic and cationic
polyelectrolytes. Trends in Colloid and Interface
Science VI. Springer.
Gao, S., Cao, R., Yang, C. 2008. Dye–polyoxometalate
composite films: Self-assembly, thermal and
photochemical properties. Journal of colloid and
interface science, 324, 156-166.
Gomes, P. J., Ribeiro, P. A., Shaw, D., Mason, N. J.,
Raposo, M. 2009. UV degradation of deoxyribonucleic
acid. Polymer Degradation and Stability, 94, 2134-
2141.
Impert, O., Katafias, A., Kita, P., Mills, A., Pietkiewicz-
Graczyk, A., Wrzeszcz, G. 2003. Kinetics and
mechanism of a fast leuco-Methylene Blue oxidation by
copper (II)–halide species in acidic aqueous media.
Dalton Transactions, 348-353.
Li, X., Sevilla, M. D., Sanche, L. 2003. Density functional
theory studies of electron interaction with DNA: Can
zero eV electrons induce strand breaks? Journal of the
American Chemical Society, 125, 13668-13669.
Mansuri-Torshizi, H., Ghadimy, S., Akbarzadeh, N. 2001.
Synthesis, characterization, DNA binding and cytotoxic
studies of platinum (II) and palladium (II) complexes of
the 2, 2'-bipyridine and an anion of 1, 1-
cyclobutanedicarboxylic acid. Chemical and
pharmaceutical bulletin, 49, 1517-1520.
Martinez, R., Chacon-Garcia, L. 2005. The search of DNA-
intercalators as antitumoral drugs: what it worked and
what did not work. Current medicinal chemistry, 12,
127-151.
Oliveira Jr, O.N., He, J., Zucolotto, V., Balasubramanian,
S., Li, L., Nalwa, H., Kumar, J., Tripathy, S. 2002.
Handbook of polyelectrolytes and their applications.
American Scientific, New York.
Oliveira Jr, O. N., Raposo, M., Dhanabalan, A. 2001.
Langmuir–blodgett and self-assembled polymeric
films. Nalwa, HS Handbook of Surfaces and Interfaces
of Mateirals, 4, 1-63.
Spencer, W., Sutter J. R. 1979. Kinetic study of the
monomer-dimer equilibrium of methylene blue in
aqueous solution. Journal of Physical Chemistry, 83,
1573-1576.
Teoule, R. 1987. Radiation-induced DNA damage and its
repair. International Journal of Radiation Biology, 51,
573-589.
Wang, Y., Liu, Y., Yang, H., Wang, H., Shen, H., Li, M.,
Yan, J. 2010. An investigation of DNA-like structured
dye-sensitized solar cells. Current Applied Physics, 10,
119-123.
Methylene Blue - A Trendy Photosensitizer in Medicine and in Solar-Energy Conversion Systems
385
