
Protocol Adapter: A Reusable Solution to Interoperability and
Integration Issues in mHealth Data-collection Systems
Alexandru Serbanati, Marcello Morena and Laura Lancia
Consorzio Roma Ricerche, Via Giacomo Peroni 130, Rome, Italy
Keywords: Medical Device, Data Collection, Interoperability, mHealth, Machine-to-Machine, Bluetooth.
Abstract: Healthcare has been changing in the last years due to several inputs, the main ones being moving from
assistive to preventive care and the introduction of patient-centric care models. In support to this tendency,
the number of consumer mobile applications for remote healthcare delivery is rapidly increasing and the use
of mobile medical sensor devices is also following. Standardization in the domain of data collection for
mHealth is still moving its first steps and, as a consequence, those who aim at developing remote healthcare
solutions must face significant problems related to the heterogeneity of sensor devices. In general, issues
related to low interoperability and low code-reusability of data collection software in mHeatlh severely limit
further developments in this sector. These issues have been addressed thanks to the Protocol Adapter which
provides a single, uniform interface for both the collection of rich data and the management of medical
devices. This article gives an overview of this component and its development process in order to provide a
better understanding of its value when integrated in mHealth applications. After an introduction to the state
of the art, the requirements for the data collection in mHealth systems are discussed. The design phase is
then described along with the final architectural solution and the features of this free, open source
implementation for Android are discussed. Finally, future works on the Protocol Adapter are discussed in
the hope to attract the interest of device producers and of mHealth developers.
1 INTRODUCTION
In a context where the patient base is growing along
with the rising of the population average age,
prevention and management of (multiple,
coexisting) chronical diseases has an increased
importance in the proposed healthcare models. As a
result, in many countries worldwide, cost-
effectiveness has been one of the main drivers in the
changes that are still undergoing in this sector
(Health and Human Services, 2011).
Another trend in this field is the focus that has
been placed on patient-centric approaches aiming at
moving the treatment context from the hospital
recovery to locations more comfortable for the
patients (Fass, 2007, and Eurobarometer, 2007).
Even WHO in its 2016-2026 roadmap envisions a
shift toward “outpatient and ambulatory care” in an
effort of reorienting the model of care (WHO, 2015).
Like in many other domains, ICT supported
these changes in many ways and, as a result, new
models for care delivery have been developed. It has
been demonstrated that “ICT-based services for
domiciliary care improve quality of life for older
people and carers, access to qualified long-term care,
and the integration of health and social care
services” (Carretero et al, 2012).
1.1 Problem Statement and Objectives
The solutions envisaged by mHealth aim to leverage
the connectivity and processing capabilities of
mobile devices to provide the relevant carer figures
with fresh and complete clinical data collected from
patients without imposing their presence in the clinic
for a reduced stress and increased comfort of the
same. To this purpose, a great variety of medical
devices that sense different clinical parameters and
communicate with different communication
protocols are available on the market. Medical
devices in the context of this paper are intended
sensor devices that can sense clinical parameters and
which are small-sized and ergonomic in order to
enable patients to wear or transport them easily.
To integrate such heterogeneous devices in
mHealth applications, often the only solution is to
550
Serbanati, A., Morena, M. and Lancia, L.
Protocol Adapter: A Reusable Solution to Interoperability and Integration Issues in mHealth Data-collection Systems.
DOI: 10.5220/0005845605500560
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 5: HEALTHINF, pages 550-560
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
develop vertical, device-specific modules for the
management of the connection and of the data
collection, increasing the architecture complexity
and reducing the part of the application that could be
re-used.
This paper describes the design of a framework
for data-collection to be used in mHealth mobile
platforms that aims at avoiding these issues by
providing a public, well-defined, well-documented
and reusable interface for interacting with medical
devices.
The result is the Protocol Adapter (PA), a
modular and extendable software architecture that
provides a single management and data collection
interface for several, heterogeneous medical devices.
In this way, connectivity aspects are separated from
business logic and GUI design so that
implementation efforts can concentrate on these
aspects rather than on integrating the communication
with medical devices.
1.2 Structure of the Paper
In the following chapters the design process is
described and the resulting architecture of the PA is
discussed. In Chapter 2 an overview of the state of
the art that was taken as starting point is described: a
brief overview of the reference architectures used in
mHealth is provided in order to understand the role
and importance of sensor medical devices in the “big
picture”. In Chapter 3 the requirements are discussed
and in Chapter 4 the analysis and design phases are
addressed. In Chapter 5 the resulting architecture
and its implementation in Android are described. In
Chapter 6 possible future work topics will be
introduced and a brief highlight of the most
important achievements completes the article.
The suggested audience is mainly ICT
professionals, i.e. developers, software analysts and
designers, system architects, etc.
2 BACKGROUND AND STATE OF
THE ART
Under the drivers of scalability, domain-wide
coherence, interoperability and the appeal of
increasing development efficiency, the need for an
open and widely accepted architecture for mHealth
was identified long ago (Estrin and Sim, 2010).
Despite the fact that standardization efforts are
still to bear fruit, when analysing mHealth
applications, common architectural solutions can be
identified and scientific and industrial effort is
invested in this field.
2.1 Architectural Reference for
mHealth
In (GSMA, 2012) a good overview of the
architectural characteristics of mHealth applications
is provided. It is also interesting to note that
similarities exist with the reference architecture for
mobile cloud computing (Dinh et al., 2013). Both
foresee the presence of a mobile device that acts as
sink and processor for the raw data coming from the
sensor and, at the same time, as an Internet gateway
for the sensors device.
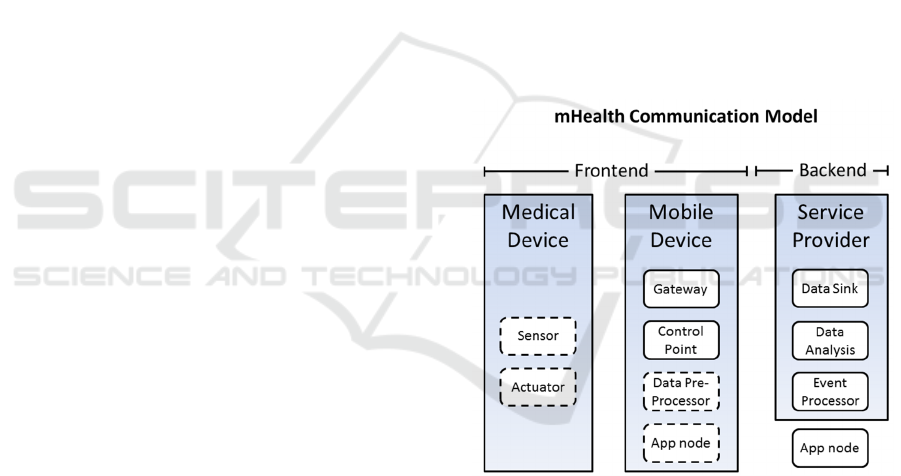
The Communication Model of mHealth
applications can be viewed as a specialization of the
Internet of Things (IoT) one. In Figure 1 the
components of the model, based on and extended
from the Communication Model from the IoT-A
project (Walewski, 2011) are shown for reference
and divided by the base platform they run on.
Figure 1: mHealth communication model components.
Dashed lines represent optional components.
It is important to note that other conventions
exist. One relevant example is the term gateway:
from the initial meaning related to protocol
adaptation at network (NWK) layer level, the
concept evolved to include security, management,
data aggregation and even application-specific
features (ITU-T, 2014). In the mHealth context
however, all these functionalities are provided by the
mobile device, which indeed is often called gateway.
With the evolution of the capabilities of such
devices into smartphones, the mobile devices also
came to host application-level software in order to
Protocol Adapter: A Reusable Solution to Interoperability and Integration Issues in mHealth Data-collection Systems
551

enable the interaction between the distributed
mHealth application and the user.
The data collection flow usually has the
following steps in mHealth systems:
1. raw data is collected by the means of sensors
embedded in medical devices (also called
sensor devices sometimes);
2. the raw data is forwarded (generally)
wirelessly to the gateway device, e.g. a
mobile or a smartphone;
3. the data can be pre-processed locally and can
be displayed for the user’s benefit on the
display of the mobile (if the mobile is also an
application node),
4. the collected data is then sent by the gateway
over the Internet to a data sink in the
backend;
5. the data is filtered, aggregated and/or stored;
6. the resulting information is made available to
users over the web/private network as
services;
7. finally, an event processor notifies users that
subscribed to specific events or clinical alerts.
The users of mHealth systems range from
patients and care personnel (i.e. medical staff,
relatives of the patient or informal carers) to other
ICT systems of the healthcare ICT environment in a
machine-to-machine (M2M) point of view.
The information flow for control and
management functions will not be investigated since
it is very specific to the examined application.
2.2 Peripheral Connections
While many technologies and standards exist for
connecting medical devices to the mobile devices,
generally data exchange happens either through a
Wireless Personal Area Network (WPAN), or
through a Wireless Local Area Network (WLAN).
At the moment of writing this paper, almost all
mobile devices are equipped with different versions
of Bluetooth and Wi-Fi interfaces for what concerns
WPAN and WLAN respectively.
An overview of the available communication
technologies and relative standardization activities
can be found in (mHISS, 2013). The majority of the
medical devices used in mHealth solutions uses
Bluetooth technology though for communication.
When designing medical sensor devices, the choice
between using Bluetooth or Wi-Fi is based on the
bandwidth and range requirements of the medical
sensor device. If the Bluetooth technology can
satisfy them, then it is preferable to use it instead of
the Wi-Fi.
The reasons behind this choice are that 1.)
Bluetooth power consumption is much lower (Lee et
al., 2007) than Wi-Fi for peer-to-peer connections,
2) Wi-Fi either needs a network infrastructure which
Bluetooth doesn’t or 3) in ad-hoc mode, Wi-Fi
interaction is less user-friendly than the Bluetooth
one, which was designed for this specific scenario
with usability in mind.
Moreover, one of the main drawbacks of
Bluetooth, its low speed compared to Wi-Fi, is no
more an issue since v3.0 and later implementations
can also include the High Speed (HS) optional
feature which enables handover to the alternate Wi-
Fi MAC/PHY in order to achieve high data rates
(Bluetooth SIG, 2009).
3 SYSTEM REQUIREMENTS
The PA development was based on requirements
that resulted from a refinement process that
consisted in three steps.
The starting point was obviously the business
goal described in the project charter: to design a
software component for mobile platforms that would
enable mobile application developers to easily
integrate the communication and the management of
the vast majority of mobile medical devices
available today off-the-shelf. A first discussion on
these requirements led to implications on the
architectural constraints related to scalability.
Licensing policy was also taken into account and
open source release was decided in accord with
company policies and in order to achieve the favour
initial distribution and take-up.
In a second step, stakeholder requirements were
gathered. For the above reasons, in the PA project,
users are of two types: developers and medical
personnel (as well as patients). While one could
argue that the end users eventually are the patients
and the medical personnel, but we thought that it
was right to include the developers too since they
will be the first users of the results of the project
both when integrating the PA implementation for
collecting medical data and when they would use the
PA architecture to extend existing implementations.
In fact in the requirement elicitation process, use
case scenarios were developed for both categories.
The PA was developed in the frame of the FI-
STAR project, as one of the components of the
platform frontend. As such, the PA development
team could leverage the use case models and
generally the requirements documentation of the
pilots of the FI-STAR project in order to derive
SmartMedDev 2016 - Special Session on Smart Medical Devices - From Lab to Clinical Practice
552

functional requirements. In particular, six pilots used
medical devices and their documentation helped a
lot in getting a good understanding of the usage
context.
In the following we provide a brief, narrative
outline of the system requirements, highlighting the
requirements that were identified during the later
stages.
3.1 Functional Requirements
Starting from the previously described business goal
statement, functional requirements for the PA were
initially derived from existing FI-STAR pilot
documentation. Since this project is centred on this
topic, a reference model for communication
interoperability based on the work from Tolk (Tolk
et al., 2007) was adopted. Figure 2 shows where the
PA impacted for the achievement of communication
interoperability.
Figure 2: Interoperability reference model and impact of
the Protocol Adapter.
As the aforementioned use case models and
medical requirements were too loosely defined,
further work towards the definition of the more
technical functional requirements was necessary.
An initial set of 32 portable medical devices that
measured 11 clinically relevant variables (SpO2,
respiratory rate, spirometry, heart rate, blood
pressure, pulse, body temperature, ECG, weight,
acceleration, blood glucose level) was collected
thanks to the support of about 15 partner
organizations.
While the ultimate aim is to support all the (types
of) devices from the aforementioned set, only a
subset was chosen for actual employment in the FI-
STAR pilots, based on the requirements of the
clinical partners. The PA architecture had to
mandatorily support these devices as a high priority
requirement.
The identified functional requirements were:
to support (also with implementations) all
device types used by FI-STAR pilots;
to support all interaction patterns between the
mobile and the medical device;
to collect clinical data from all devices used
in the use cases;
to support the largest number of device
models on the market;
to provide the clinical data in a single format;
to provide information about the status of
medical device;
to manage the connection with the medical
device.
As we believe that this technical details might be
interesting for the reading audience, the relevant
ones will be detailed in the following.
3.1.1 Interaction Patterns
Medical sensor devices are very heterogeneous for
what concerns the way they operate. The difference
regard:
the operations that need to be performed in
order to establish a communication channel
(such as Bluetooth pairing or physical
attachment);
the sequence of operations that establish the
communication channel itself: whether the
mobile or the medical device is the initiator,
i.e. which one initiates the communication;
the role of the devices: related to which is the
server and which the client; please note that
this is not necessary related to the initiator
role;
the duration of the connection: some devices
maintain it until the application decides to
terminate it, others automatically cut it off as
soon as they have sent the data;
the necessity to perform a setup before
operation: more complex devices need to be
provided with operational parameters upon
connection in order to start operating;
the need to send a command in order to start
data acquisition; this command can also
include information about the measurements
to be performed;
the way devices send the data: some devices
send data automatically and immediately
after performing the measurement, while
others send the data only after the proper
command is received.
All of these differences have to be taken into
Protocol Adapter: A Reusable Solution to Interoperability and Integration Issues in mHealth Data-collection Systems
553

account. Moreover, information about the status of
the device must be provided to the application.
3.1.2 Data Collection
Data collection, i.e. the process by which the values
measured by the medical sensor devices are
collected by the mobile devices, can use several
different communication technologies as well as
different protocols. The main concern, derived
directly from the business goal, is that the Protocol
Adapter must be able to integrate all these options to
collect data.
This requirement set is also related to the
available implementation options: while the design
drives the implementation, when designing it is
important to know what are the implementations
constraints. In our case, we had to deal with the fact
that many medical devices used (sometimes
proprietary) protocols for which closed libraries
existed. These protocols – and the relative libraries –
reach different levels of the communication stack.
The initial set of devices used different
communication solutions at PHY/MAC layer level:
Bluetooth, audio jack, and Wi-Fi. However, even in
the Bluetooth device set, different Bluetooth Profiles
were used: Health Device Profile (HDP) (Bluetooth
SIG, 2012), Smart Bluetooth (Bluetooth SIG, 2010),
or open and closed protocols over SPP. Moreover, it
was decided to keep the most generic approach
possible in order to be able to support all kind of
existing devices and to make it possible to easily
provide support even to future ones.
3.1.3 Data Provision
All the previously illustrated differences have
implications on the syntactic and semantic level. The
following requirements were gathered with the help
of the developers of the pilots and are related to the
medical needs of the pilot use cases:
the data has to be provided with a uniform
syntax and semantics, despite differences in
the single device protocol,
the data has to be provided as soon as it
arrives,
the data has to be as rich as possible: no
information has to be omitted and, moreover,
contextual information that regards the device
should be sent along with the measurement.
3.2 Non-functional Requirements
Some constraints derived mainly from the high level
goals, e.g. from the project charter:
mHealth architecture compliant: the PA had
obviously to comply with the aforementioned
mHealth architecture;
FI-WARE Protocol Adapter architecture
compliant: as part of the Future Internet
programme, the PA was initially supposed to
implement the interfaces of the Protocol
Adapter component of the Internet of Things
Services Enablement (FI-WARE, 2015) FI-
WARE chapter;
the component had to be easily extensible in
the future in order to support new
technologies, maintaining at the same time
backward compatibility;
M2M and IoT readiness: while the current
mHealth architecture is slightly influenced by
the IoT one, current architectures appear to
be centralised and the M2M approach is only
considered in the backend part. The PA team
expects that this situation will evolve and that
data will be eventually provided directly “at
source”, i.e. from the mobile device, allowing
the user to really be in control of his data.
3.2.1 External Interface
For the PA, the external interface was the interface
for communicating with the application that needed
to collect the data. Such applications can be either
local or remote or, in some cases, a local application
will use the data but will also forward it to the
backend. So, no assumption could be made on
whether the consumer of the information was local,
as in the case of the gateway pre-processing the data,
or remote, i.e. in the backend environment.
Another non-functional requirement related to
the external interface was the need to reduce
complexity and increase the possibility to reuse both
knowledge and code in order to increase
development efficiency. This was both a business
goal and a developers’ need.
3.2.2 Deployment Requirements
Different operating systems have different
architectures, resulting in different resources that can
be used, different security restrictions, different way
of integrating components and communicating with
them, different best practices, and so on.
Also, both the PA implementations and the
applications that are going to use them must use the
APIs and resources provided by such platforms (i.e.
operating systems) or on APIs provided by vendors
SmartMedDev 2016 - Special Session on Smart Medical Devices - From Lab to Clinical Practice
554

which, in turn, run on the lower layers of the OS.
3.2.3 Performance and Other Requirements
The PA is meant to mediate and simplify
interactions with external devices connected to the
mobile device where the application and the PA
itself run. When integrating the PA to collect data
from a medical device, it will not degrade
significantly the performance of the system when
compared to a system directly integrating the
medical device control into the application itself.
This means for example that the PA will have to be
able to manage fast data flows as in the case of
ECG sensors. This kind of performance can also be
impacted by the number of functionalities that can
be accessed through the PA compared to the number
of functionalities provided by the device.
It was also flagged as important that product
vendors also needed to be able to extend the PA with
their own software that could be closed source or
generally subject to different licensing policies
compared to the PA.
4 DESIGN AND ARCHITECTURE
In first stance, the initial (i.e. the broadest) set of
device models and the sequences needed for their
use were considered. To this purpose, since the
interaction had to be carried out programmatically,
their development kits and protocol documentation
was analysed. Interaction models were abstracted
from this information. Several different use cases
(and models) requiring different sequences of
interaction were identified. All were taken into
account except that of a device that was designed to
communicate only with a proprietary app in order to
send the collected data directly to a third party,
predefined Internet server. It was found that several
models would be needed for representing these
device types and yet different models had subsets of
common features.
Indeed we found out that it was better to abstract
characteristics than to use monolithic models. These
characteristics are related to connection roles and
modes as well as to operational requirements. In the
following a list of such characteristics is provided:
the device acts as a connection initiator;
the device supports external configuration:
four behaviours were identified
(configuration supported only at startup,
supported at runtime, supported both at
startup and runtime, not supported);
the device supports external commands;
the device can be detected programmatically
prior to connection;
the device needs pairing (or other previous
setup) prior to connection;
the device can be disconnected
programmatically;
the development kit provides a reliable way
to know if the device is connected and
operating.
For what concerns data collection and
interoperability, it was clear that several protocols
were used by the devices on the market. The
individual syntax and semantics had thus to be
abstracted by the PA in order to provide a single
external interface for managing the data collection
from medical sensor devices.
The use of such protocols had to be supported at
different levels of the communication stack and they
guaranteed different levels of interoperability. Some
protocols were only documented, while some
devices provided data collection features through
APIs. Thus, the resulting data representation varied
from one device type to another.
The HDP device type was considered as single
type on par with device models for the purpose of
this analysis because the reference Personal Health
Device standard (i.e. the IEEE11073 standard
family) directly provides pragmatic interoperability
for all the compatible device models. Indeed
implementations of the PA that cover HDP already
have a large pool of compatible device that are
supported.
Finally, the analysis also resulted in the rejection
of some requirements. For instance, the requirement
to provide a component that implemented the FI-
WARE Protocol Adapter interface had to be dropped
because it was conflicting with the best practices for
development on mobile devices. In particular, using
NGSI-9/10 Context Management specifications
(Open Mobile Alliance, 2012) over REST
connections could not be fulfilled because
workarounds for the fact that mobile devices had
dynamic network addresses would severely impact
on the device batteries.
4.1 Notes about Architecture Design
When designing an abstract model that could
represent all types of devices, the right level of
abstraction had to be found. Emphasis was thus put
on providing a high level model with relatively few
and generic functionalities that could be easily
understood and mastered.
Protocol Adapter: A Reusable Solution to Interoperability and Integration Issues in mHealth Data-collection Systems
555

In order to understand at what level of the
interoperability stack would the PA be placed, the
available APIs and the documentation of
communication protocols was analysed. Contextual
information and descriptions of the measurements
was available and could be provided along with the
raw values communicated by the medical devices.
Moreover, the sequence of operations needed for the
proper operation of devices changed from one type
of devices to another and trying to automate this part
would increase the complexity of the PA interface.
For these reasons it was decided against trying to
provide fully-fledged pragmatic interoperability.
It was thus decided that the PA would only
provide descriptive information to the upper layers
(i.e. the application) about the device types and their
characteristics. While this information is not
sufficient to operate all devices and it is not meant to
replace the knowledge about the operation of more
complex devices, it helps in avoiding the misuse of
the PA. We take as granted that, if a developer has to
use a device that requires the sending of a command
to start the acquisition of data, this is known to the
developer.
The devices were characterized by: the ID of the
device, their serial number, the model name,
manufacturer name, the physical address of the
device and a collection of the attached sensors to the
device represented using the Sensor Model.
The sensors, in turn, were characterized by: the
name of the sensor, the name of the property
measured by the sensor and the measurement unit of
said property.
The Device and Sensor Models are meant to be
used together to describe the devices and their
sensors.
After the device model was defined, the internal
architecture of the PA had to be designed. In order to
have a single interface towards the application and
to allow third parties to expand the PA support
independently, device specific functionalities were
separated: a specific component called Device
Adapter (DA) would manage low-level, device
specific functionalities while a higher level
component, the Protocol Adapter Manager (PAM),
would provide the single point of interface with the
application and DA-management functionalities.
The implementations of the DA are required to
be able to recognize the devices that they could
manage and provide protocol adaptation for a given
type of sensor medical devices.
Moreover, in order not to limit the development
possibilities, it was also chosen to allow in principle
the existence of more DAs able to manage the same
types of devices and even to allow their
implementations to coexist on the same mobile
device: the user (human user or application) will
then have to decide which implementation should
manage which device.
The communication requirements of the devices
were analysed and a set of message types was
defined for what concerns the interaction of the
PAM with both the application and the DAs. The
majority of interactions that could be started by the
application resulted in an asynchronous feedback
from the device because the device had to perform
some physical measurement (in some cases even
with the user’s implication) and a synchronous
response could not be guaranteed. However, some
management interactions between the PAM and the
DA which don’t depend on the medical devices
could be carried out synchronously.
For what concerns the collection of data, this has
to be translated from the original format to a single
common format. In this way, applications will
receive the data structured in a uniform way despite
the different device source, the different
measurement types, the different formats that are
provided in input and so on. In turn, this will allow
developers both to use only one interpretation
routine for all the devices they used and also to reuse
existing code over different projects.
For this reason, an Observation Model based on
the information types returned by the considered
devices was defined in order to provide an uniform
information model. In the design, M2M
requirements were kept into account along with the
previous experience that the team had in the field of
data collection and IoT. This impacted for example
on the data collected from devices that provided
measurements continuously, such as for example
electrocardiographs. It was decided that in the event
in which the medical device sent streams of data,
this had to be grouped in packets as an internal
mechanism of the DA, while the PAM should not
support data streaming. One of the main reasons for
this choice was that such type of data is not
generally supported in M2M frameworks where data
is represented as a static, albeit possibly complex,
resource.
The model was meant to represent in a well-
known format every possible measurement carried
out by every possible device. It includes the name of
the property which the measurement refers to, the
measurement unit, the time of the measurement, the
duration of the measurement and a collection of raw
samples of the measured value.
SmartMedDev 2016 - Special Session on Smart Medical Devices - From Lab to Clinical Practice
556
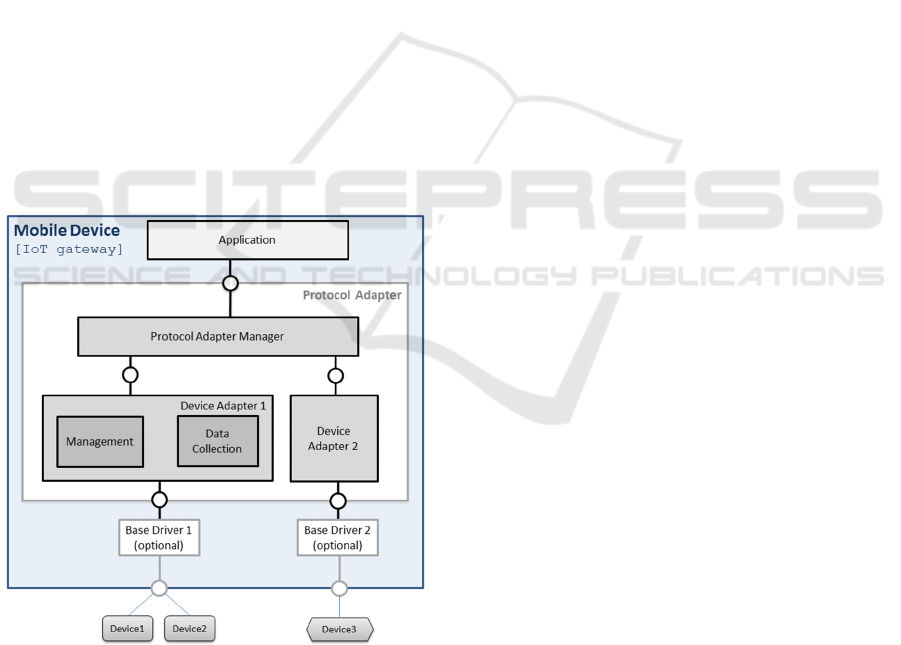
4.2 Resulting Architecture
Based upon the previously described analysis and
design, the architecture of the Protocol Adapter is
shown in Figure 3. The PAM provides an interface
to the application above and manages from an
operational and communication point of view any
number of DAs below it.
In more detail, the DA must establish (or allow
the medical sensor device to establish) a
communication channel. If needed, it then must use
this channel to send the configuration or specific
commands needed for bringing the device to an
operational status. Finally, it will provide protocol
adaptation between the device specific formats and
the single PAM format, for which it actually acts as
an abstraction layer. If vendor drivers are available
for a given device, DA designers can use them to
ease the development process.
The PAM on the other hand must discover all the
DA instances available on the system upon start and
afterwards it must manage their life cycle. It must
provide all the collected data to the application,
handling all the measurements and the events
generated by the DA. In the other way, it also has to
route the application device commands and
management inquiries to the right DA.
Figure 3: Protocol Adapter Architecture.
Also, Device, Sensor and Observation logical
models were used to represent characteristics and
functionalities of the managed devices, integrated
sensors and collected data. These models are used
together to coherently pass knowledge above these
concepts from one component to another.
5 ANDROID IMPLEMENTATION
To respect the requisites of flexibility and
expandability, it has been chosen to implement the
PA as a collection of separate Android applications
communicating via Inter Process Communication
(IPC) mechanism (Google, 2015a).
5.1 Components and Interfaces
In particular, the PA Android implementation
consists of a certain number of DA applications and
one PAM application, each of them deployed
through a specific .apk. In the following part of this
chapter DA and PAM will be used with the meaning
of implementations, i.e. Android applications, unless
explicitly stated that they are meant as models.
The two crucial design decisions about the
Android implementation were: what IPC mechanism
to choose amongst the ones offered by Android and
how to implement the discovery phase.
The choice of the right IPC mechanisms was
based on two development requirements. In first
stance, reliable communication channels between
the PAM and DAs and between the PAM and third
party applications was needed. In second stance, it
was necessary to package all the software needed for
the establishment of said channels inside a library, in
order to make the integration of the PA as simple as
possible for third party developers. In the end we
chose Android Interface Definition Languages
(AIDL). In this way, the Java implementations of the
Device, Sensor and Observation models, called
respectively DeviceDescription, SensorDescription
and Observation, could be packaged along with the
AIDL interfaces and the Capabilities class used to
represent DA properties and distributed as a library.
Also this allowed to have a reliable return value for
the methods that needed one.
This method is used so that, after a successful
binding to services, Android applications can invoke
methods of remote objects (belonging to other
Android applications) as if they were local.
At this point, two kinds of channels existed: one
that enabled the communication of the DAs with the
PAM, and the channel between the third party
application and PAM. Since we wanted bidirectional
communication for each channel, using AIDL we
created a total of four Java interfaces, two for each
channel. Every time a service is bound to, depending
on the kind of the channel, two objects
Protocol Adapter: A Reusable Solution to Interoperability and Integration Issues in mHealth Data-collection Systems
557

implementing the related interfaces are exchanged
between the application that implements the service
and the application that is bounding to it. This
allows a bidirectional communication that is carried
on in the most natural way for Java software: simply
invoking methods on objects.
Finally, the DAs and the PAM were
implemented as Android Services because, being
them software modules designed to run in the
background without a direct user interaction and to
offer APIs to third party applications, the Service
paradigm was the most appropriated.
5.2 Discovery Implementation
The discovery feature was implemented with
another Android IPC mechanism that is more
common and more lightweight than AIDL, yet less
powerful and less reliable: the Intent system
(Google, 2015b). When using Intents to send
messages between applications, there has to be a
software component inside the receiving application
called Broadcast Receiver. This component must
declare what types of Intents it is interested into and
is invoked every time that a suitable Intent is
dispatched in the system by a sender application.
However, using this facility, the sender application
can never know if the Intent has been successfully
delivered and it is impossible for the receiving
application to acknowledge or directly reply to the
received message.
In the PA, every DA implements a Broadcast
Receiver that obtains all the discovery Intents
generated by the PAM and sends back a reply Intent
(which the PAM has a Broadcast Receiver for) to
notify its presence on the system. Moreover, the DA
sends, together with the reply Intent, an object called
Capabilities; this object contains all the relevant
information about the DA itself that the PAM needs
to know in order to properly handle it.
5.3 Operation
The application binds to the Manager using facilities
included in the library and establish the bidirectional
communication channel. The PAM, upon the start,
performs the discovery process to retrieve
information about all the DAs available on the
system. Once this phase is done, the PAM activates
all the DAs that it needs by binding to their related
services and establishing a bidirectional
communication channel with every one of them. At
this point, the third party application gets notified
that the initialization phase is over. From now on,
devices that are initiators can spontaneously connect
and send data, while devices that are not initiators
can be connected to (upon request of the third party
application) and triggered to send data. At a certain
point, all types of devices will eventually be
connected. When this happens, the third party
application gets notified and receives all the details
of the newly connected device via a
DeviceDescription object. Every time a device sends
new data, this is forwarded to the third party
application encapsulated in an Observation object.
These operations continue until the third party
application is bound to the PAM. When the third
party application decides to terminate the bound
with the PAM, it will be shut down gracefully
together with all the active DAs, releasing all the
connected devices in the process.
The descriptive models of devices, sensors and
observations are implemented as java classes called
respectively DeviceDescription, SensorDescription
and Observation. They provide a well-known
structure that could be used to encapsulate
information forwarded to the PAM or to the
application.
6 CONCLUSIONS AND WAY
FORWARD
The PA is a free, open source component that
succeeds in bridging the interoperability gap that
exists at the low level in the mHealth
communication domain. The development process
and the resulting components with their features
have been presented in this paper, along with their
Android implementation, in the hope to raise the
interest of two stakeholders that the authors believe
to be essential to the success of the PA: the mHealth
application developers and device manufacturers.
The Android version of the PA source is
available at https://github.com/theIoTLab/ along
with DAs for HDP devices and for the Zephyr
BioHarness 3 device. Other DAs have been
developed but could not be published due to
licensing issues.
6.1 Further Developments
EHealth is a domain that is rapidly growing,
continuously providing new solutions and
integration with traditional healthcare. To keep the
pace, the PA will need to adequate to major
reference architectures, standards and best practices.
SmartMedDev 2016 - Special Session on Smart Medical Devices - From Lab to Clinical Practice
558

For this reason, while the PA is already a FI-
STAR component, we would also like to integrate
the PA in the FI-WARE architecture as a native
Generic Enabler.
Another development thread is related to
supporting new device models and device types. For
example, the development of an Android DA
implementation for Smart Bluetooth is under
evaluation.
Last but not least, we think that the security
aspect of the interaction with the devices should be
improved. Currently, for example, there is no way
for the gateway (or for the application running on
the gateway) to authenticate the medical device.
Unfortunately, this cannot be implemented on the
PA side only, because it also requires support from
the medical devices. Yet, we believe these features
to be critical from a long term perspective since they
are required for securely implementing the support
of mHealth actuators.
ACKNOWLEDGEMENTS
The research on the Protocol Adapter was funded in
the frame of the FP7 FI-PPP Phase II project Future
Internet Social and Technological Alignment in
Healthcare (FI-STAR) under grant no. 604691.
REFERENCES
Bangash, J. I., Abdullah, A. H., Anisi, M. H., and Khan,
A. W., 2014. A survey of routing protocols in wireless
body sensor networks. In Sensors vol.14, n. 1, pp.
1322-1357. MDPI.
Bluetooth SIG, 2012. Health Device Profile Specification.
Available online from
https://developer.bluetooth.org/TechnologyOverview/
Pages/HDP.aspx.
Bluetooth SIG, 2009. Bluetooth High Speed Technology,
available online at https://www.bluetooth.org/en-
us/specification/adopted-specifications.
Bluetooth SIG, 2010. Bluetooth Smart (Low Energy)
Technology. Available online at
https://developer.bluetooth.org/TechnologyOverview/
Pages/BLE.aspx.
Carretero, S., Stewart, J., Centeno, C., Barbabella, F.,
Schmidt, A., Lamontagne-Godwin, F., Lamura, G.,
2012. Can Technology-based Services support Long-
term Care Challenges in Home Care? Analysis of
evidence from social innovation good practices across
the EU CARICT Project Summary Report.
Publications Office of the European Union.
Dinh, H. T., Lee, C., Niyato, D., and Wang, P., 2013. A
survey of mobile cloud computing: architecture,
applications, and approaches. In Wireless
communications and mobile computing, vol. 13, n. 18,
pp. 1587-1611. Wiley Online Library.
Estrin D, and Sim, I., 2010. Open mHealth architecture: an
engine for health care innovation. In Science, vol. 330,
n. 6005, pp.759–760.
Eurobarometer, Special, 2007. Health and long-term care
in the European Union." Special Eurobarometer 283.
Fass, L., 2007. Patient-centric healthcare, IET.
FI-WARE, 2015. Internet of Things Services_Enablement.
Available online at
http://forge.fiware.org/plugins/mediawiki/wiki/fiware/
index.php/Internet_of_Things_(IoT)_Services_Enable
ment.
Fricker, S. A., Thuemmler, C., Mival, O, 2013. Technical
Requirements and Architecture Report including Open
Call Requirements. EC FP7 FI-STAR (604691)
project deliverable D1.1.
Google, 2015a. Bound Services, Developer’s
documentation, available online at
http://developer.android.com/guide/components/bound
-services.html#Creating.
Google, 2015b. Intents and Intent Filters, Developer’s
documentation, available online at
http://developer.android.com/guide/components/intent
s-filters.html.
GSMA, 2012. Connected Mobile Health Devices: A
Reference Architecture.
ITU-T, 2013. Common requirements and capabilities of a
gateway for Internet of things applications,
Recommendation ITU-T Y.2067. In Global
Information Infrastructure, Internet Protocol Aspects
And Next-Generation Networks, ITU.
Lee, J. S., Su, Y. W., and Shen, C. C., 2007. A
comparative study of wireless protocols: Bluetooth,
UWB, ZigBee, and Wi-Fi. In Industrial Electronics
Society, 2007. IECON 2007. 33rd Annual Conference
of the IEEE, pp. 46-51. IEEE.
mHIMSS, 2013. mHIMSS Roadmap: Standards and
Interoperability. Available online at
http://www.himss.org/ResourceLibrary/genResourceD
etailPDF.aspx?ItemNumber=21293.
Open Mobile Alliance (OMA), 2012. NGSI Context
Management, Version 1.0.
Schweitzer, J., and Synowiec, C., 2012. The economics of
eHealth and mHealth. In Journal of health
communication, vol. 17, n. sup1, pp. 73-81. Taylor &
Francis.
Walewski, J.W., (ed.), 2011. Initial Architectural
Reference Model for IoT. EC FP7 IoT-A (257521),
project Deliverable Document D1.2.
World Health Organization (WHO), 2015. Reorienting the
model of care, In WHO global strategy on people-
centred and integrated health services, WHO Press.
Tolk, A., Diallo, S., Turnitsa C., 2007. Applying the levels
of conceptual interoperability model in support of
integratability, interoperability, and composability for
system-of-systems engineering. In Journal of
Systemics, Cybernetics and Informatics, vol 17, n. 5,
pp. 65-74.
Protocol Adapter: A Reusable Solution to Interoperability and Integration Issues in mHealth Data-collection Systems
559

U.S. Department of Health and Human Services, March
2011, 2011 Report to Congress: National Strategy for
Quality Improvement in Health Care. Available online
at http://www.ahrq.gov/workingforquality/nqs/
nqs2011annlrpt.htm.
SmartMedDev 2016 - Special Session on Smart Medical Devices - From Lab to Clinical Practice
560
