
Spectral-luminescent Properties of Silver Clusters Formed in
Ion-exchanged Antimony-doped Photo-thermo-refractive Glasses
Yevgeniy Sgibnev, Nikolay Nikonorov and Alexander Ignatiev
Department of Optical Information Technologies and Materials, ITMO University,
Birzhevaya line 4, 199034, St. Petersburg, Russian Federation
Keywords: Photo-thermo-refractive Glass, Ion Exchange, Silver Clusters, Silver Nanoparticles.
Abstract: Photo-thermo-refractive glasses are now attractive material for developing various elements and devices of
photonics. Influence of antimony oxide content in the photo-thermo-refractive glass composition and
subsequent heat treatment temperature on the spectral-luminescent properties of silver non-metal clusters
and metal nanoparticles formed with low-temperature ion exchange method were studied. Silver clusters in
ion-exchanged Sb-doped photo-thermo-refractive glasses reveal broadband and intense emission in the
visible and near infrared ranges. Absolute quantum yield of luminescence reaches 63% (λ
ex
=365 nm), which
opens up new prospects for using such materials as phosphors for white LEDs and down-convertors for
solar cells.
1 INTRODUCTION
Silver clusters, which are subnanosized aggregates
consisting of several silver atoms and/or ions, in
glasses are well known (Bourhis et al. 2013;
Dubrovin et al. 2014) to have an intense broadband
luminescence in the visible. Today glasses with
luminescent silver clusters were proposed to be used
as phosphors for white LEDs (A. S. Kuznetsov et al.
2013), luminescence down-shifting cover glasses for
solar cells (Cattaruzza et al. 2015), and optical data
storage media (Klyukin et al. 2014). However, low
quantum efficiency of luminescence of silver
clusters stabilized in various glass hosts, which does
not exceed 35% at room temperature up to now
(Sgibnev et al. 2016; Cattaruzza et al. 2015;
Kuznetsov et al. 2012), limits their industrial
applications.
Properties of some silver clusters well studied in
solutions (Díez et al. 2012), zeolites (De Cremer et
al. 2009), and solid rare gas matrices (Harbich et al.
1990; Félix et al. 1999). However, it is impossible in
principle to grow a certain kind of silver clusters in
glasses. Thereby, it should be remembered that
different types of silver clusters with various
structural and optical properties always coexist in a
glass host.
At present, photo-thermo-refractive (PTR)
glasses that are already used widely in photonics
(Nikonorov et al. 2001) can be classified as
polyfunctional materials combining, in themselves,
the properties of several monofunctional materials
such as the photorefractive, holographic, laser,
plasmonic, photostructurable, and ion exchangeable
ones. Bragg gratings based on PTR glasses are used
as laser line narrowing and stabilizing filters,
spectral and spatial filters, Raman filters,
compressors for fs- and ps-lasers, spectral beam
combiners, high power beam splitters, etc.
(Andrusyak et al. 2009).
As known, PTR glass is multicomponent
sodium-zinc-aluminosilicate one containing
halogens (fluorine and bromine) and doped with
antimony, cerium, and silver. Mechanisms of
photochemical reactions and subsequent
nanocrystallization in PTR glasses were studied in
detail in (Dubrovin et al. 2016).
It should be noted that, owing to the low
solubility of silver in silicate glasses (the order of
10
19
cm
−3
for soda-lime ones), the maximum
possible silver oxide concentration in PTR glasses
does not exceed 0.1% mol. However, thin layer with
high concentration of silver can be easily formed
with low-temperature ion exchange method. The ion
exchange (IE) technology is known (Tervonen et al.
2011; Ramaswamy and Srivastava 1988) to be based
on substituting one kind of alkali cations (usually
Sgibnev Y., Nikonorov N. and Ignatiev A.
Spectral-luminescent Properties of Silver Clusters Formed in Ion-exchanged Antimony-doped Photo-thermo-refractive Glasses.
DOI: 10.5220/0006212303730377
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Na
+
) in glass for another one (Li
+
, K
+
, Rb
+
, Cs
+
) or
transition metal ions (Ag
+
, Cu
+
, Tl
+
) from a salt
melt.
As mentioned above, the PTR glass composition
contain antimony that in the form of Sb
3+
can act as
a donor of electrons for silver ions. In this work,
dependence of spectral-luminescent features of
silver clusters and nanoparticles formed with low-
temperature ion exchange in PTR glasses depending
on antimony content was investigated. Moreover,
influence of heat treatment temperature on the
optical properties of silver clusters and nanoparticles
was studied as well.
2 EXPERIMENTAL
In order to investigate the effect of antimony ions
alone on the formation of silver clusters and
nanoparticles in PTR glasses, other dopants (such as
silver and cerium oxides and also bromine) should
be excluded from the glass compositions. Glass
blocks of samples based on the 14Na
2
O–3Al
2
O
3
–
5ZnO–71.5SiO
2
–6.5F (mol. %) matrix of typical
PTR glasses doped with different concentrations of
Sb
2
O
3
were synthesized. Batch antimony oxide
content of synthesized PTR matrix-based glass
samples was 0, 0.002, 0.004, and 0.01 mol. %,
(hereafter referred as GS0, GS2, GS4, and GS10,
respectively). The glass synthesis was conducted in
an electric furnace at 1500 °C in the air atmosphere
using the platinum crucibles and mechanical stirrer.
The glass transition temperature of the glasses
measured with STA 449 F1 Jupiter (Netzsch)
differential scanning calorimeter was found to be
464±3 °С. Planar polished samples 1 mm thick were
prepared for further investigation.
Silver ions were incorporated into the above PTR
matrix-based glass samples with ion exchange
method. The samples were immersed in a bath with
a melt of nitrate mixture 5AgNO
3
/95NaNO
3
(mol.
%) at temperature T
IE
=320 °C for 15 minutes. A
gradient layer enriched by silver ions about 10 μm
thick was formed due to replacing the Na
+
ions in
glass by Ag
+
ones from a salt melt. The ion-
exchanged samples were then heat-treated at
different temperatures (250−500 °С) for 15 hours.
The absorption spectra of the samples were recorded
with double-beam spectrophotometer Lambda 650
(Perkin Elmer). The registration of emission spectra
excited by UV light at 365 nm and absolute quantum
yield measurements were carried out inside
integrated sphere with Photonic Multichannel
Analyzer (PMA-12, Hamamatsu) at room
temperature. The measurement error for the absolute
quantum yield (AQY) was ±1%.
3 RESULTS AND DISCUSSIONS
3.1 Influence of PTR Glass
Composition
A long-wavelength shift of the UV edge of strong
absorption with respect to its initial location was
observed for all ion-exchanged glass samples. The
shift results from the absorption envelope of Ag
+
ions with maximum around 225 nm caused by the
interionic 4d
10
→4d
9
5s
1
transitions (Sgibnev et al.
2013). Weak luminescence assigned to different
silver clusters were occurred in the visible range
after the IE. In the course of the IE process, due to a
great increase in the concentration of Ag
+
ions,
chemical equilibrium of a reaction:
2Ag
+
+ Sb
3+
↔ 2Ag
0
+ Sb
5+
(1)
shifts to the right side in compliance with Le
Chatelier principle (Jenkins 2008). Subsequent
aggregation of silver atoms and ions through
chemical reactions:
Ag
0
+ Ag
+
→ Ag
2
+
(2)
Ag
0
+ Ag
0
→ Ag
2
(3)
Ag
0
+ Ag
2
→ Ag
3
(4)
and similar ones results in formation of different
non-metal silver clusters. Thus, growth of silver
clusters takes place in the course of the IE and lead
to occurrence of weak luminescence in the visible.
Increase in Sb
3+
ions content in the PTR glass
composition increases rate of the chemical reaction
(1), i.e. rate of reducing silver ions Ag
+
to the atomic
state Ag
0
. Thereby, formation kinetics of silver
clusters and nanoparticles is determined by
concentration of reducing agent (Sb
3+
ions) in the
initial glass, which is proved experimentally by
absorption spectra of PTR glass samples (Fig. 1).
Additional absorption bands were not observed
in PTR glass sample GS0 with no antimony (i.e.
silver remains in the GS0 glass in the ionic form).
Absorption spectra of Sb-doped PTR glasses shows
additional absorption bands centered at 350 and
420 nm. The long-wavelength band corresponds to
the surface plasmon resonance (SPR) of silver
nanoparticles (Schasfoort and Tudos 2008). The
other one with maximum in the UV assigned to non-
metal silver clusters (Ag
n
, n≥2). Increase in
antimony oxide content results in growth of the
amplitude and changing relation of the bands.
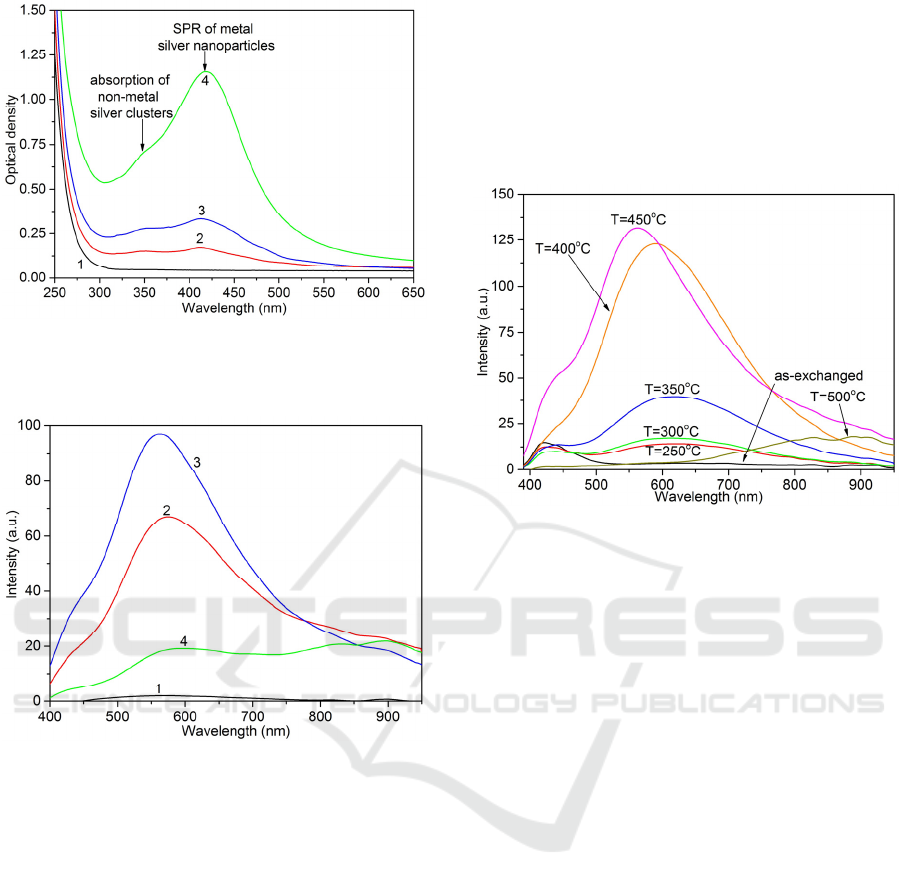
Figure 1: Optical density spectra of PTR glasses GS0-
GS10 (1)-(4), respectively, after the IE and subsequent
heat treatment at 500 °С.
Figure 2: Emission spectra (λ
ex
= 365 nm) of PTR glasses
GS0-GS10 (1)-(4), respectively, after the IE and
subsequent heat treatment at 500 °С.
Fig. 2 shows emission spectra of the PTR glasses
after the IE and subsequent heat treatment at
T=500 °С for 15 h. PTR glass GS0 demonstrates
weak luminescence in the visible related to small
amount of silver clusters formed by trapping
electrons from glass impurities by silver ions. In Sb-
doped PTR glasses intense and broadband
luminescence of silver clusters in the range 400-
950 nm was observed. Emission peak in the visible
occurs around 560 nm and can be assigned to Ag
3
clusters that demonstrate emission bands peaked at
560 and 616 nm under 362 nm excitation in the solid
argon matrix (Fedrigo et al. 1993). Luminescence in
the visible quenches with increasing antimony oxide
concentration. The luminescence quenching results
from both decreasing amount of emitting centers due
to transformation «cluster → nanoparticle» and
absorption of the emission by silver nanoparticles.
3.2 Influence of the Heat Treatment
Temperature
As it was shown in (Simo et al. 2012; Sgibnev et al.
2016) heat treatment temperature has a significant
impact on properties of silver aggregates in ion-
exchanged glasses.
Figure 3: Emission spectra (λ
ex
= 365 nm) of the ion-
exchanged PTR matrix-based glass GS10 prior to any heat
treatment and after the heat treatment at temperatures 250-
500 °С (temperature values are indicated on the graph).
Fig. 3 clearly shows substantial effect of the heat
treatment temperature on shape and intensity of the
emission spectra of silver clusters formed in Sb-
doped PTR glass. Shape of the emission spectra of
the samples heat-treated at temperatures 250-350 °C
remains unchanged, which evidences that
concentration of silver clusters increases, while
relation of different kinds of luminescent centers
keeps constant. The emission maximum is located at
620 nm that coincides with emission of Ag
3
clusters
(Fedrigo et al. 1993). Further rising heat treatment
temperature up to 400 and 450 °C results in
significant increase in luminescence intensity and
blue shift of the emission peak. The blue shift can be
assign to formation in glass host of Ag
4
clusters that
in the argon matrix are characterized by the UV
absorption bands at wavelengths up to 405 nm and
the main emission band at 458 nm (Félix et al.
1999). Absorption of the luminescence of silver
clusters by metal nanoparticles formed in the ion-
exchanged layers after the heat treatment at 500 °C
leads to changing emission color from yellowish
white to deep red (Fig. 4). The NIR emission in PTR
glasses can be assigned to large silver clusters Ag
n
(n>4) remaining in the glass after heat treatment at
500 °C.
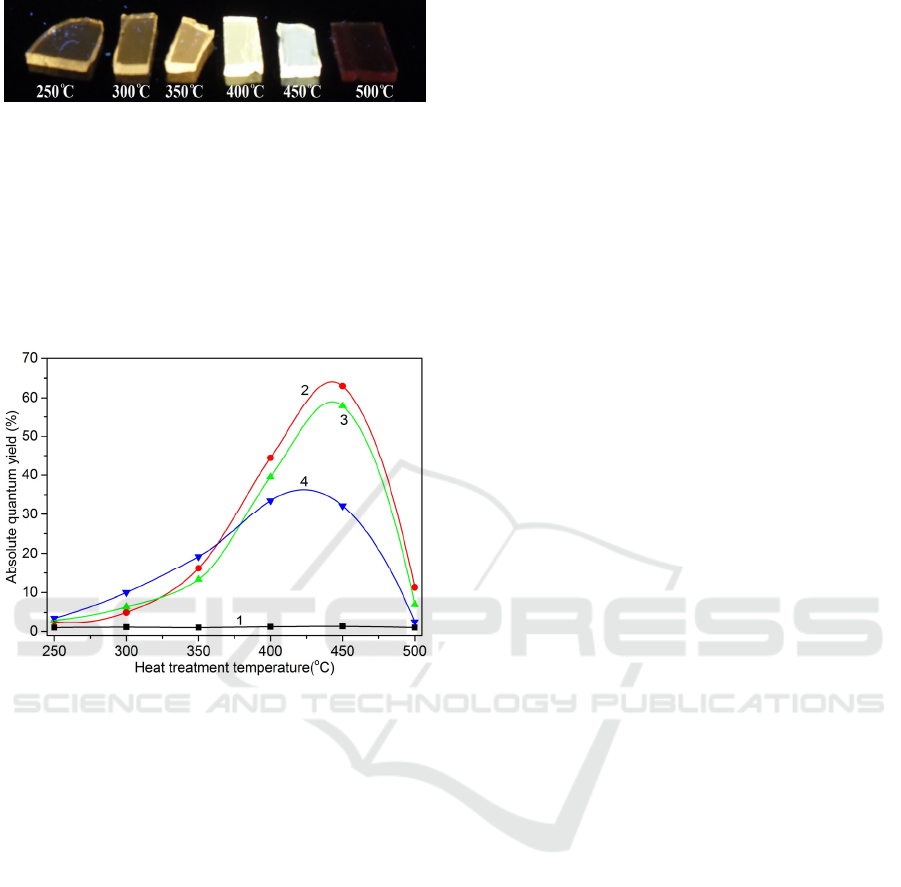
Figure 4: Photo of the PTR glass GS10 samples subjected
to the IE and subsequent heat treatment at temperatures
250-500 °С.
Absolute quantum yield (AQY) allows to
estimate efficiency of converting UV light in the
visible range, that is why it is an important
parameter for industrial applications of glasses with
silver clusters as luminescence down shifting
material or phosphor.
Figure 5: Dependence of AQY magnitudes for ion-
exchanged PTR glasses GS0-GS10 (1)-(4), respectively,
on the temperature of the subsequent heat treatment.
The AQY magnitudes of all as-exchanged and
heat-treated at 250 °C glass samples do not exceed
4%. The subsequent heat treatment did not lead to
any increase in the AQY magnitude for antimony-
free GS0 glass (Fig. 5). Heat treatment of the Sb-
doped PTR glass samples at 300 and 350 °C causes
weak growth of AQY up to 6-9% and 12-18%,
respectively. In the course of the heat treatment of
antimony-doped samples at 400 and 450 °C the
concentration of silver clusters increases
dramatically and, hence, the AQY magnitudes
increase as well. A strong enough AQY dependence
on the antimony oxide concentration emerges at
these temperatures. For example, the AQY
magnitudes achieved after the heat treatment at
450 °C for 15 h are 63%, 59% and 32% for GS2,
GS4, and GS10 glasses, respectively. A further
increase in the heat treatment temperature up to
500 °C leads, for all Sb-doped ion-exchanged glass
samples, to a decrease in their AQY magnitudes
compared to those of samples heat-treated at 450 °C.
This results from decreasing the amount of emitting
centers and absorption the emission of silver clusters
by silver nanoparticles.
Thus, heat treatment temperature determines
color, intensity, and quantum yield of the
luminescence of silver clusters dispersed in surface
layers of ion-exchanged Sb-doped PTR glasses.
4 CONCLUSIONS
Influence of antimony oxide content in the PTR
glass composition and subsequent heat treatment
temperature on the spectral-luminescent properties
of silver non-metal clusters and metal nanoparticles
formed in the PTR glasses with low-temperature ion
exchange method were studied. Antimony ions Sb
3+
are the donor of electrons for silver ions Ag
+
and
play a key role in growth of silver luminescent
clusters and plasmonic nanoparticles. Silver clusters
in Sb-doped PTR glasses reveal broadband and
intense emission in 400-950 nm range. Metal
nanoparticles in ion-exchanged PTR glasses are
formed only after subsequent heat treatment at
temperature higher than the glass transition one and
quench the luminescence. Absolute quantum yield
magnitude of luminescence in Sb-doped PTR
glasses with silver clusters can be as high as 63%. It
opens up new prospects for using such materials as
phosphors for white LEDs and down-convertors for
solar cells.
ACKNOWLEDGEMENTS
Research was funded by Russian Science
Foundation (Agreement #14-23-00136).
REFERENCES
Andrusyak, O. et al., 2009. Spectral Combining and
Coherent Coupling of Lasers by Volume Bragg
Gratings. IEEE Journal on Selected Topics in
Quantum Electronics, 15(2), pp.344–353.
Bourhis, K. et al., 2013. Formation and thermo-assisted
stabilization of luminescent silver clusters in
photosensitive glasses. Materials Research Bulletin,
48(4), pp.1637–1644.
Cattaruzza, E. et al., 2015. Ag
+
↔Na
+
ion exchanged
silicate glasses for solar cells covering: Down-shifting
properties. Ceramics International, 41(5), pp.7221–
7226.
De Cremer, G. et al., 2009. Characterization of

fluorescence in heat-treated silver-exchanged zeolites.
Journal of the American Chemical Society, 131(8),
pp.3049–3056.
Díez, I. et al., 2012. Blue, green and red emissive silver
nanoclusters formed in organic solvents. Nanoscale,
4(15), pp.4434–7.
Dubrovin, V.D. et al., 2014. Luminescence of silver
molecular clusters in photo-thermo-refractive glasses.
Optical Materials, 36(4), pp.753–759.
Dubrovin, V.D., Ignatiev, A.I. and Nikonorov, N. V.,
2016. Chloride photo-thermo-refractive glasses.
Optical Materials Express, 6(5), p.1701.
Fedrigo, S., Harbich, W. and Buttet, J., 1993. Optical
response of Ag2, Ag3, Au2, and Au3 in argon
matrices. The Journal of Chemical Physics, 99(1993),
pp.5712–5717.
Félix, C. et al., 1999. Fluorescence and excitation spectra
of Ag4 in an argon matrix. Chemical Physics Letters,
313(1-2), pp.105–109.
Harbich, W. et al., 1990. Deposition of mass selected
silver clusters in rare gas matrices. J. Chem. Phys.,
93(12), pp.8535–8543.
Jenkins, H.D.B., 2008. Chemical Thermodynamics at a
Glance, Blackwell Publishing, Oxford, 1
st
edition.
Klyukin, D. a et al., 2014. Luminescence quenching and
recovering in photo-thermo-refractive silver-ion doped
glasses. Optical Materials, 38, pp.233–237.
Kuznetsov, A. S. et al., 2013. Ag nanocluster
functionalized glasses for efficient photonic
conversion in light sources, solar cells and flexible
screen monitors. Nanoscale, 5(21), pp.10065–75.
Kuznetsov, A. S. et al., 2012. Quantum yield of
luminescence of Ag nanoclusters dispersed within
transparent bulk glass vs. glass composition and
temperature. Applied Physics Letters, 101(25).
Nikonorov, N. V. et al., 2001. Influence of Glass
Composition on the Refractive Index Change upon
Photothermoinduced Crystallization. Glass Physics
and Chemistry, 27(3), pp.241–249.
Ramaswamy, R. V. and Srivastava, R., 1988. Ion-
Exchanged Glass Waveguides: A Review. Journal of
Lightwave Technology, 6(6), pp.984–1000.
Schasfoort, R.B.M. and Tudos, A.J., 2008. Handbook of
surface plasmon resonance, RSC Publishing.
Sgibnev, E.M. et al., 2013. Effects of silver ion exchange
and subsequent treatments on the UV-VIS spectra of
silicate glasses. I. Undoped, CeO
2
-doped, and (CeO
2
+
Sb
2
O
3
)-codoped photo-thermo-refractive matrix
glasses. Journal of Non-Crystalline Solids, 378,
pp.213–226.
Sgibnev, Y.M., Nikonorov, N.V. and Ignatiev, A. I., 2016.
Luminescence of silver clusters in ion-exchanged
cerium-doped photo-thermo-refractive glasses.
Journal of Luminescence, 176, pp. 292–297.
Simo, A. et al., 2012. Formation mechanism of silver
nanoparticles stabilized in glassy matrices. Journal of
the American Chemical Society, 134(45), pp.18824–
18833.
Tervonen, A., West, B.R. and Honkanen, S., 2011. Ion-
exchanged glass waveguide technology: a review.
Optical Engineering, 50, p.71107.
