
Augmenting Guideline-based CDSS with Experts’ Knowledge
Nekane Larburu
1,2
, Naiara Muro
1,2
, Iván Macía
1,2
, Eider Sánchez
3
, Hui Wang
4
, John Winder
5
,
Jacques Boaud
6,7
and Brigitte Séroussi
7,8
1
eHeatlh and Biomedical Applications, Vicomtech-IK4, Donostia-San Sebastian, Spain
2
Biodonostia, Donostia-San Sebastian, Spain
3
NARU, Donostia-San Sebastian, Spain
4
Computer Science Research Institute, Ulster University, Newtownabbey, U.K.
5
Institute of Nursing and Health Research, Ulster University, Newtownabbey, U.K.
6
AP-HP, DRCD, Paris, France
7
Sorbonne Universités, UPMC Univ. Paris 06, INSERM, Université Paris 13, Sorbonne Paris Cité,
UMR S 1142, LIMICS, Paris, France
8
AP-HP, Hôpital Tenon, DSP, Paris, France
Keywords: Evidence Based Medicine, Breast Cancer, Computer Interpretable Clinical Guidelines, CDSS.
Abstract: Over the past years, clinical guidelines have increasingly become part of the clinical daily practice in order to
provide best available Evidence-Based-Medicine services. Hence, their formalization as computer
interpretable guidelines (CIG) and their implementation in clinical decision support systems (CDSSs) are
emerging to support clinicians in their decision making process and potentially improve medical outcomes.
However, guideline compliancy in the clinical daily practice is still “low”. Some of the reasons for such low
compliance rate are (i) lack of a complete guideline to cover special clinical cases (e.g. oncogeriatric cases),
(ii) absence of parameters that current guidelines do not consider (e.g. lifestyle) and (iii) absence of up-to-
date guidelines due to lengthy validation procedures. In this paper we present a novel method to build a CDSS
that, besides integrating CIGs, stores experts’ knowledge to enrich the CDSS and provide best support to
clinicians. The knowledge includes new evidence collected over time by the systematic usage of CDSSs.
1 INTRODUCTION
In order to offer the best available care, medical
practice adopts the Evidence-Based-Medicine (EBM)
principle, defined as “the conscientious, explicit and
judicious use of current best evidence in making
decisions about care of individual patients” (Sackett
et al., 1996). In the 90s clinical practice guidelines
(CPGs) start to appear as rigorous evaluations of
different clinical activities that improved the clinical
practice and developed health care processes
(Grimshaw and Russell, 1994), so that clinicians
could follow EBM. However, clinicians still found
barriers to adhere to CPGs (Cabana MD et al., 1999).
Some of these barriers were lack of awareness, lack
of familiarity, lack of agreement, lack of outcome
expectancy or the inertia to previous practice. These
barriers are still valid in the current practice.
In order to overcome some of the main obstacles,
during the last decade multiple CPGs have been
formalized in an electronic way, i.e. computer
interpretable guidelines (CIG), and applied in Clinical
Decision Support Systems (CDSSs) (B. Séroussi et
al., 2013). Nevertheless, it was discovered that CPGs
still have limitations. For instance, in the context of
breast cancer (BC) some factors such as elderly
patients, multifocal tumours, occurrence of
micrometastasis on lymph-node and patient choice
are causes of CPGs non-compliance (Chéreau et al.,
2011; Landercasper et al., 2006; Lebeau et al., 2011;
B. Séroussi et al., 2013).
In this paper we present a method to acquire
expert knowledge in order to develop a knowledge-
augmented guideline-based CDSS. It results in the
development of new tools to support clinicians on
their decision making process for cases that have low
evidence (e.g. oncogeriatric cases) or where other
aspects (e.g. patient preferences) are crucial.
370
Larburu N., Muro N., Macà a I., Sà ˛anchez E., Wang H., Winder J., Boaud J. and SÃl’roussi B.
Augmenting Guideline-based CDSS with Expertsâ
˘
A
´
Z Knowledge.
DOI: 10.5220/0006213903700376
In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2017), pages 370-376
ISBN: 978-989-758-213-4
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

The rest of this paper is organized as follows.
Section 2 presents the state of the art on CIG
compliance. Section 3 presents the method to
augment the guideline-based CDSS with expert´s
knowledge. Section 4 presents the application of such
method in an EU project, DESIREE, developed in the
context of breast cancer. Section 5 proposed a short
discussion on the presented method and Section 6
concludes the paper and gives some future work lines.
2 STATE OF THE ART
2.1 Guideline Compliance
Variations in medical practices have been observed
for decades, questioning the quality of care (Mercuri
and Gafni, 2011). CPGs compliance is one of the
primary performance measures to assess the quality
of medical practice. McGlynn et al. (McGlynn et al.,
2003) reported that 54.9% of the studied patient
population received CPGs’ based recommended care,
which vary from 10% to 78%. In their work they
reported that for BC 75.7% were consistent with
recommended care, based on 9 quality indicators.
Other studies also demonstrated suboptimal guideline
compliance levels in BC (Adegboyega et al., 2015;
Landercasper et al., 2006; Lebeau et al., 2011;
Wöckel et al., 2010). The published levels of
guideline compliance range from 12% (Lebeau et al.,
2011) to 100% (Adegboyega et al., 2015), depending
on the definition of guideline compliance and the
level of abstraction of the guideline. For instance,
Wöckel et al. (Wöckel et al., 2010) reported 80% of
adherence to German-S3-BC guideline for surgery
and for hormone therapy, and 71% for chemotherapy,
indicating different compliance levels for the
different components of the care plan. Similarly,
Lebeau et al. (Lebeau et al., 2011) reported high level
of guideline compliance, but also said that
“management of non-metastatic BC was fully
compliant (considering jointly 20 quality criteria)”.
2.2 Causes Associated with Guideline
Non-Compliance
The causes of variations in care delivery are
multifactorial. A review by Flottorp et al. (Flottorp et
al., 2013) identified a list of 51 determinants of
practice grouped in seven domains: guideline factors,
individual health professional factors, patient factors,
professional interactions, incentives and resources,
capacity for organisational change, and social,
political, and legal factors.
However, effective guideline-based CDSSs
(Beeler et al., 2014; Roshanov et al., 2013) provide a
framework for logging non-compliance cases and
learn from them. As demonstrated by Séroussi et al.
(B. Séroussi et al., 2013), guideline compliance
increases by using guideline-based CDSS.
Additionally, Bouaud et al. (Bouaud and Séroussi,
2011) determined the main factors related with CPG
non-compliance and reported the distribution of non-
compliance causes. Here, we list these causes
reported in (Bouaud and Séroussi, 2011):
Patient preferences: When patients receive
more complete information about the benefits
and risks of different treatment options, the
patients made their own active, informed
decisions (Leonard et al., 2011). This decision
is influenced by their personal preferences.
Evolution of medical knowledge: CPG
knowledge may not consider latest scientific
publications and clinical essays, and hence,
they may lag behind ‘last’ evidence (B.
Séroussi et al., 2013). This may include that
‘new’ parameters are not being considered in
the applied guidelines.
Specific situations: Rare situations that require
specific clinical research are also a cause of
non-compliance. For example, in BC scenarios
shown in (Parks et al., 2012; Schnitt, 1998; B
Séroussi et al., 2013), microinvasion,
neadjuvant situations and oncogeriatry
conditions are the main causes that lead into
non-compliance situations.
Medical choices: One of the main cause of
non-compliance is a medical decision that is
prioritized over the guideline recommendation.
For example, in (Bouaud and Séroussi, 2011),
the study reported that BC multidisciplinary
staff meetings’ choice (i.e. breast units choice)
is the main reason reported as the cause for
CPG non-compliance.
Others: Finally, it may be other reasons that
lead into CPG non-compliancy that do not
belong to any of the previously reported causes.
Some studies provide tools to support clinicians
in understanding the reasons of non-compliancy
(Hussain et al., 2007). Others exploit the stored
patient information to predict patient worsening and
prevent potential emergencies (Colantonio et al.,
2008). Yet, there is no evidence that all the
information related to the whole decision making
process (such as additional patient data, the decision
criteria for giving a specific treatment and patient
Augmenting Guideline-based CDSS with Expertsâ
˘
A
´
Z Knowledge
371
outcomes) is stored and exploited over time to enrich
the CDSS and provide better decision support to
decision makers in prospective cases.
3 METHOD TO AUGMENT
GUIDELINE-BASED CDSS
Here we present a method that enables the
exploitation of the implicit knowledge used in a
decision making process. The method is presented in
the following subsections: Section 3.1 presents the
starting point, which applies the clinical guideline
model, Section 3.2 presents the second stage, which
describes the acquisition process of experts’
knowledge and Section 3.3 presents how such
experts’ knowledge is exploited.
3.1 Clinical Guideline Model
As discussed in Section 1, CPGs are intended to
optimize patient care. Therefore, in this initial stage a
clinical guideline model is developed. The clinical
guideline model incorporates (i) different guidelines
based on users’ needs, (ii) updated clinical guidelines
or studies, so that the provided recommendations
correspond to the latest available evidence, and
detects (iii) potential inconsistencies that could be
reflected on the implemented guidelines.
3.2 Experts’ Knowledge Acquisition
The second stage of this method focuses on experts’
knowledge acquisition and storage.
We developed a flexible solution that enables the
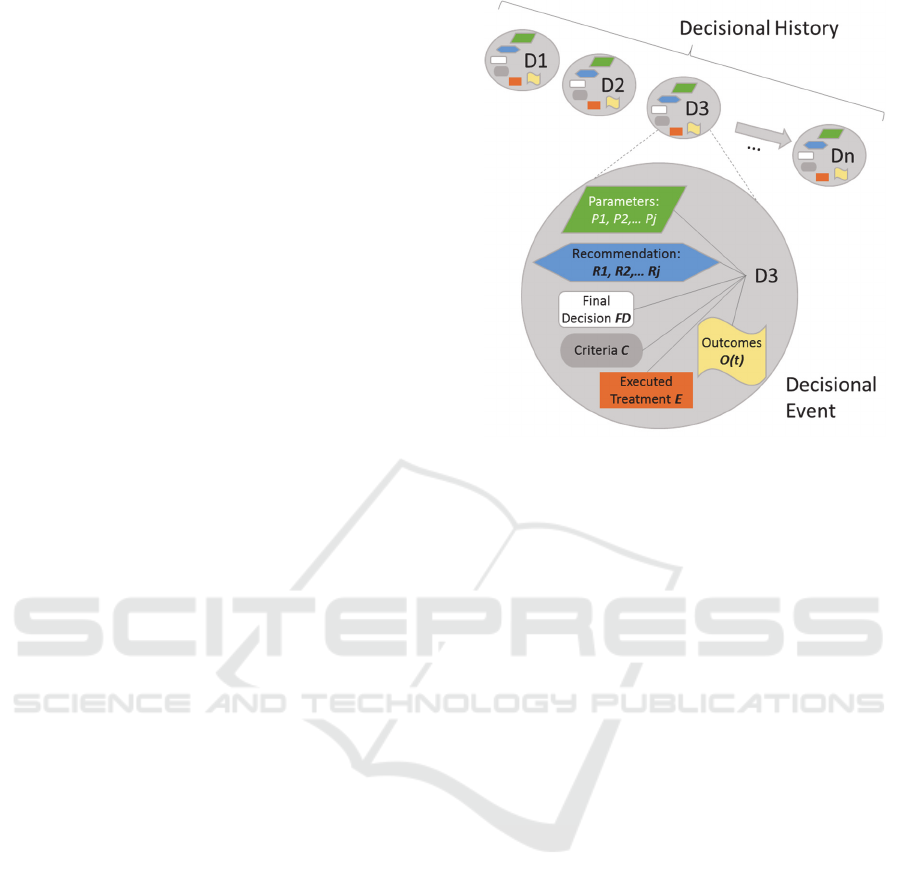
storage of each decisional event (Figure 1). Each
decisional event reflects all the rationality for taking
a decision and the consequences of such decision.
Hence, we define the decisional event as
,
,
,,, where (i) P
i
is a set of
patient parameters involved in the decision-making
process, (ii) R
j
is a set of clinical conditions (e.g.
rules) wherein such parameters have been analysed,
which results in a set of recommendations (iii) FD is
a final decision that is taken by the decision maker,
(iv) C
k
is a set of criteria for which the final decision
is made (which could be a patient parameter), (v) E is
the executed treatment (usually, same as the final
decision) and (vi) O(t) is the health outcomes of a
patient measured over time t (e.g. (“ICHOM –
International Consortium for Health Outcomes
Measurement,” n.d.)).
Figure 1: Decisional event and decisional history.
As shown in Figure 1, the storage of decisional
events over time lead into a decisional history. The
decisional history is later used to retrieve conclusions
or discover new knowledge (Section 3.3).
3.3 Experts Knowledge Exploitation
Here we present the three usages of this decisional
history: (i) recommendations assessment, (ii) patient
similarity based recommendations and (iii)
knowledge discovery to extend the knowledge base.
3.3.1 Recommendation Assessment
As presented by (Fox et al., 2009), “the current
guideline development lifecycle does not provide
appropriate tools to assess their impact on clinical
practice”. The proposed system is able to evaluate the
decisions taken quantitatively (e.g. based on the
number of times the recommendation was followed)
and qualitatively (e.g. based on the patient outcomes
– when the results are successful or match the defined
decision criteria). This quantitative and qualitative
measurements are presented to clinicians during the
clinical decision making process to provide enriched
information of the given recommendations.
3.3.2 Patients’ Similarities
The system also applies similarity features between
different patients and their results to support
clinicians in their decision making process. For that,
the system uses different metrics to determine which
(clinical) parameters have higher impact when
HEALTHINF 2017 - 10th International Conference on Health Informatics
372

determining how similar a patient could be to a
retrospective patient (e.g. age range, TNM
classification etc.). In cases where the benefits and
harms of a specific treatment are not clear, clinicians
are able to consult previous similar patient cases and
their outcome before taking a decision. The previous
patient cases could be specific patient cases, or
‘model’ cases that summarise n past cases.
3.3.3 Knowledge Discovery
The experience acquired from the decisional history
may enable different type of knowledge acquisition.
Here we present the two types of knowledge
considered in our research.
Firstly, the information from a large number of
cases enables the adjustment of CPGs and protocols´
clinical conditions, e.g. in a form of a rule. For that,
the scope of the criteria is redefined based on the
cases where the given guideline-based
recommendation is being followed with successful
results. This is implemented using machine learning
techniques. For example, if a decision criterion is
parameter ∈ 0.5, 1.5, after applying machine
learning techniques the system recognizes that the
recommendation is being successful only when ∈
0.8, 1.3. It also detects when a parameter, not
previously included into the clinical condition for the
decision making process, is determinant and should
be part of the existing decision rule.
Secondly, large number of non-compliant cases
with good or better results than the ones that follows
the CPGs may lead into an extension of the CPGs´
clinical conditions (e.g. rules) by generating ‘new’
branches. This ‘new’ branches may include
recommendations (treatment actions) that are not
considered in the available CPGs (e.g. “clinical trial”)
or may include recommendations that are in the
CPGs, but that are not considered for the given case.
This cases could make the knowledge base either
more restrictive when a rule becomes more precise,
but also could extend it with further procedures that
were not included in the knowledge base.
In both adjustment and extension cases, in order
to include the ‘new’ knowledge into the CDSS, the
system verifies if the outcomes are positives and
informs clinicians about its potential usage. If
approved, this knowledge is included into the
knowledge base for the CDSS (Figure 2).
Nevertheless, the system provides the information of
the recommendation source. This way clinicians are
aware if the recommendation is guideline based or
created automatically by the system based on the
recorded experience or patient similarity properties.
4 DESIGN IN DESIREE
This study is being performed in the context of a
European H2020 project, named DESIREE. In this
section we present DESIREE project (Section 4.1)
and the data flow diagram that represents our
methodology within DESIREE (Section 4.2).
Figure 2: Data flow diagram.
4.1 DESIREE
DESIREE aims to provide decision support on the
available therapy options by incorporating evidence
based guidelines and experience from previous cases
and outcomes. Hence, DESIREE goes beyond the
limitations of existing guideline-based decision
support systems. Such a system targets breast cancer
(BC) cases, which is one of the most common and
most deadly type of cancer affecting woman in the
EU countries, with more than 460,000 new cases and
130,000 deaths in 2012 (Ferlay et al., 2013).
The users of such system are medical domain
experts involved on breast units (BU) where patients’
diagnosis and treatment decisions are taken. Hence,
the system goal is to support BU during their weekly
meetings in their multidisciplinary decision making
process by providing not only CPGs based decision
support, but also additional information extracted
from previous cases over time.
4.2 DESIREE
The data flow diagram presented in Figure 2 is a high
level representation of DESIREE platform. Since
DESIREE is developed in the context of BC, in the
depicted figure, Breast Units (BU) are the clinical
experts that make the final decision. Here, we
describe each block presented in Figure 2, omitting
the blocks that correspond to the data presented in
Section 3.2.
Augmenting Guideline-based CDSS with Expertsâ
˘
A
´
Z Knowledge
373

Narrative Guidelines: In our methodological
approach, the starting point is the analysis of
representative and narrative CPGs used in BC
care.
Computer Interpretable Guidelines:
Knowledge engineers extract the relevant
information from CPGs and formalize it in a
CIG. This covers the recommendations given
by guidelines for primary BC in several stages
of the whole treatment till the patient is
discharged. Hence, the CIG consider the
previous treatments and the outcomes of them
for the coming decision making action.
Knowledge: The knowledge database stores
knowledge from the CIG or from the decisional
history exploitation´s “new” knowledge.
Rule-based Engine: The rule-based engine is
able to generate recommendations having as
input the structured knowledge. Then, if patient
data fulfils the clinical condition, the rules are
fired and the engine generates one or more
recommendations.
Knowledge Discovery: Based on a large set of
information stored in the decisional history, the
system is capable of retrieving knowledge as
discussed in Section 3.3.
5 DISCUSSION
The presented method overcomes some limitations of
current guideline-based CDSS by providing enriched
recommendations and additional information to
clinicians in order to support them best in their
decision making process. For that, we develop a new
information structure based on decisional events. A
decisional event stores the whole set of information
used in the decision making process, including the
consequences of the final decision, such as patient´s
outcomes (e.g. quality of life).
Here we present some of the potential benefits and
limitations of the proposed method. Firstly, the
system promotes the usage of CPGs. Additionally, it
assess the impact of the guidelines on clinical practice
(Section 3.3.1), which is one of the critical factors
detected by (Fox et al., 2009). Secondly, the system
flexibility enables the storage of additional valuable
information, such as the decision criteria, that could
be used to adjust or/and extend the clinical conditions
of the given protocols and CPGs over time (Section
3.3.3). This way it helps overcoming some of the
limitations of current CPGs presented in Section 2,
such as the impact of specific situations. This
diverges from the work done in other projects, such
as MobiGuide (Larburu et al., 2015), where the
guidelines are customized and made context-aware
beforehand during the knowledge engineering phase,
and not over time depending on previous cases.
Neither we focus on the discovery of temporal rules
from time-stamped data, like in (Sacchi et al., 2007).
Our study aims to discover rules from previous cases
tracking each case to assess the outcomes and
considering further information often not taken into
account in current CPGs, such as the implicit
knowledge of clinicians. Finally, the presented
method combines both the CPGs and the knowledge
generated automatically by the system based on their
experience, which overcomes the requirements
expressed by clinicians in (Miranda-Mena et al.,
2006): “clinicians want a system that combines the
protocol (or CPG) and their proper knowledge to
suggest treatments”.
6 CONCLUSIONS & FUTURE
WORK
The hypothesis of this research is that such approach
is more useful for clinicians, which expect a dynamic
system that not only considers available CPGs and
protocols, but also a system that is able to learn from
the stored information over time to provide enriched
decision support system.
In future work we aim to present among others the
following points: (i) the tools used to convert the
information acquired by experience into knowledge
to extend and adjust the CPGs and protocols; (ii) a
digital patient model ontology used for the CDSS, and
particularly for similarity purposes; (iii) the
methodology to assess the recommendations
applying different metrics (survival rate, overall well-
being, physical functioning etc.); and (iv) the
validation of the system in a representative number of
patients and the results.
ACKNOWLEDGEMENTS
This project has received funding from the European
Union’s Horizon 2020 research and innovation
programme under grant agreement No 690238.
REFERENCES
Adegboyega, T.O., Landercasper, J., Linebarger, J.H.,
Johnson, J.M., Andersen, J.J., Dietrich, L.L., Driscoll,
HEALTHINF 2017 - 10th International Conference on Health Informatics
374

C.D., Raghavendra, M., Madadi, A.R., Al-Hamadani,
M., Vang, C.A., Marcou, K.A., Hudak, J., Go, R.S.,
2015. Institutional review of compliance with NCCN
guidelines for breast cancer: lessons learned from real-
time multidimensional synoptic reporting. J. Natl.
Compr. Cancer Netw. JNCCN 13, 177–183.
Beeler, P.E., Bates, D.W., Hug, B.L., 2014. Clinical
decision support systems. Swiss Med. Wkly. 144,
w14073.
Bouaud, J., Séroussi, B., 2011. Revisiting the EBM
decision model to formalize non-compliance with
computerized CPGs: results in the management of
breast cancer with OncoDoc2. AMIA. Annu. Symp.
Proc. 2011, 125–134.
Cabana MD, Rand CS, Powe NR, et al, 1999. Why don’t
physicians follow clinical practice guidelines?: A
framework for improvement. JAMA 282, 1458–1465.
Chéreau, E., Coutant, C., Gligorov, J., Lesieur, B., Antoine,
M., Daraï, E., Uzan, S., Rouzier, R., 2011. Discordance
with local guidelines for adjuvant chemotherapy in
breast cancer: reasons and effect on survival. Clin.
Breast Cancer 11, 46–51.
Colantonio, S., Conforti, D., Martinelli, M., Moroni, D.,
Perticone, F., Salvetti, O., Sciacqua, A., 2008. An
intelligent and integrated platform for supporting the
management of chronic heart failure patients, in: 2008
Computers in Cardiology. Presented at the 2008
Computers in Cardiology, pp. 897–900.
Ferlay, J., Steliarova-Foucher, E., Lortet-Tieulent, J.,
Rosso, S., Coebergh, J.W.W., Comber, H., Forman, D.,
Bray, F., 2013. Cancer incidence and mortality patterns
in Europe: Estimates for 40 countries in 2012. Eur. J.
Cancer 49, 1374–1403.
Flottorp, S.A., Oxman, A.D., Krause, J., Musila, N.R.,
Wensing, M., Godycki-Cwirko, M., Baker, R., Eccles,
M.P., 2013. A checklist for identifying determinants of
practice: a systematic review and synthesis of
frameworks and taxonomies of factors that prevent or
enable improvements in healthcare professional
practice. Implement. Sci. IS 8, 35.
Fox, J., Patkar, V., Chronakis, I., Begent, R., 2009. From
practice guidelines to clinical decision support: closing
the loop. J. R. Soc. Med. 102, 464–473.
Grimshaw, J.M., Russell, I.T., 1994. Achieving health gain
through clinical guidelines II: Ensuring guidelines
change medical practice. Qual. Health Care 3, 45–52.
Hussain, S., Abidi, S.R., Abidi, S.S.R., 2007. Semantic
Web Framework for Knowledge-Centric Clinical
Decision Support Systems, in: Artificial Intelligence in
Medicine. Presented at the Conference on Artificial
Intelligence in Medicine in Europe, Springer, Berlin,
Heidelberg, pp. 451–455.
ICHOM – International Consortium for Health Outcomes
Measurement [WWW Document], n.d. URL
http://www.ichom.org/ (accessed 1.3.17).
Landercasper, J., Dietrich, L.L., Johnson, J.M., 2006. A
Breast Center review of compliance with National
Comprehensive Cancer Network Breast Cancer
guidelines. Am. J. Surg. 192, 525–527.
Larburu, N., Schooten, B. van, Shalom, E., Fung, N.,
Sinderen, M. van, Hermens, H., Jones, V., 2015. A
Quality-of-Data Aware Mobile Decision Support
System for Patients with Chronic Illnesses, in
Knowledge Representation for Health Care, Lecture
Notes in Computer Science. Springer International
Publishing, pp. 126–139.
Lebeau, M., Mathoulin-Pélissier, S., Bellera, C., Tunon-de-
Lara, C., Daban, A., Lipinski, F., Jaubert, D., Ingrand,
P., Migeot, V., REPERES Group, 2011. Breast cancer
care compared with clinical Guidelines: an
observational study in France. BMC Public Health 11,
45.
Leonard, R., Ballinger, R., Cameron, D., Ellis, P.,
Fallowfield, L., Gosney, M., Johnson, L., Kilburn, L.S.,
Makris, A., Mansi, J., Reed, M., Ring, A., Robinson,
A., Simmonds, P., Thomas, G., Bliss, J.M., 2011.
Adjuvant chemotherapy in older women (ACTION)
study - what did we learn from the pilot phase? Br. J.
Cancer 105, 1260–1266.
McGlynn, E.A., Asch, S.M., Adams, J., Keesey, J., Hicks,
J., DeCristofaro, A., Kerr, E.A., 2003. The Quality of
Health Care Delivered to Adults in the United States.
N. Engl. J. Med. 348, 2635–2645.
Mercuri, M., Gafni, A., 2011. Medical practice variations:
what the literature tells us (or does not) about what are
warranted and unwarranted variations. J. Eval. Clin.
Pract. 17, 671–677.
Miranda-Mena, T.G., Benítez U., S.L., Ochoa, J.L.,
Martínez-Béjar, R., Fernández-Breis, J.T., Salinas, J.,
2006. A knowledge-based approach to assign breast
cancer treatments in oncology units. Expert Syst. Appl.
31, 451–457.
Parks, R.M., Lakshmanan, R., Winterbottom, L., AL
Morgan, D., Cox, K., Cheung, K.-L., 2012.
Comprehensive geriatric assessment for older women
with early breast cancer – a systematic review of
literature. World J. Surg. Oncol. 10, 88.
Roshanov, P.S., Fernandes, N., Wilczynski, J.M., Hemens,
B.J., You, J.J., Handler, S.M., Nieuwlaat, R., Souza,
N.M., Beyene, J., Van Spall, H.G.C., Garg, A.X.,
Haynes, R.B., 2013. Features of effective computerised
clinical decision support systems: meta-regression of
162 randomised trials. BMJ 346, f657.
Sacchi, L., Larizza, C., Combi, C., Bellazzi, R., 2007. Data
mining with Temporal Abstractions: learning rules
from time series. Data Min. Knowl. Discov. 15, 217–
247.
Sackett, D.L., Rosenberg, W.M.C., Gray, J.A.M., Haynes,
R.B., Richardson, W.S., 1996. Evidence based
medicine: what it is and what it isn’t. BMJ 312, 71–72.
Schnitt, S.J., 1998. Microinvasive carcinoma of the breast:
a diagnosis in search of a definition. Adv. Anat. Pathol.
5, 367–372.
Séroussi, B., Laouénan, C., Gligorov, J., Uzan, S., Mentré,
F., Bouaud, J., 2013. Which breast cancer decisions
remain non-compliant with guidelines despite the use
of computerised decision support? Br. J. Cancer 109,
1147–1156.
Augmenting Guideline-based CDSS with Expertsâ
˘
A
´
Z Knowledge
375

Séroussi, B., Laouénan, C., Gligorov, J., Uzan, S., Mentré,
F., Bouaud, J., 2013. Which breast cancer decisions
remain non-compliant with guidelines despite the use
of computerised decision support? Br. J. Cancer 109,
1147–1156.
Wöckel, A., Kurzeder, C., Geyer, V., Novasphenny, I.,
Wolters, R., Wischnewsky, M., Kreienberg, R., Varga,
D., 2010. Effects of guideline adherence in primary
breast cancer--a 5-year multi-center cohort study of
3976 patients. Breast Edinb. Scotl. 19, 120–127.
HEALTHINF 2017 - 10th International Conference on Health Informatics
376
