
Translating a Clinical Workflow into a Computer-executable Model
User Needs Discovery, Challenges and Lessons Learned
Ana Leitão, Jennifer Caffarel and Erin Stretton
Royal Philips - Research, High Tech Campus 34, Eindhoven, The Netherlands
Keywords: User Requirements, Healthcare IT, User Confrontation, Clinical Pathways.
Abstract: Getting to technical requirements from user input is already a hard task in an environment where the workflow
processes are very well defined. When trying to extract a unique process from the users of such a variable
work environment such as a healthcare institution can be very challenging. In this paper, we share our
experience with extracting user requirements from clinical users by presenting the specific example of
transforming workflows into models that can then be used as part of an IT solution to support workflow
guidance. Here we present not only some of our main challenges when approaching different institutions and
professionals with different roles, but also some of the methods we find most useful to establish
communication and extract as much relevant information possible. In the end we explain some of the
differences between a workflow as explained by the users and a computer–executable model and how to make
the connection between the two.
1 INTRODUCTION
In 2004 Ash et Al. said that “we should strive to have
a national system of Electronic Health Record (EHR)
that can share information on any patient in any
health care setting”. While some institutions have
the financial capabilities to have the latest technology
which allows them to automate and make a wide
range of tasks digital, there are still institutions who
depend very highly on paper, especially when it
comes to documenting clinical processes and
pathways.
From our learnings this persistence on the use of
paper to document clinical processes and pathways
within a hospital is not only driven by lack of
resources and financial capabilities to go digital but
in many cases, paper is seen as an easier alternative
to implement than modifying the IT system to support
such tasks. Also, the IT systems are still having
negative impact on the work of the clinicians by
increasing the documentation time and being
incompatibility with clinical workflow. This leads to
a higher amount of interruptions in the medical work
and system-introduced errors in patients care.
(Ologeanu-Taddei, R. et al., 2015; Jamoom, E. W. et
al., 2016)
Another limitation of paper supported processes is
that although this may make the administrative task
of collecting most relevant information in one
location easier, they generally do not aid the clinical
users to adhere to the recommended steps in a
pathway in real-time – they mainly serve as a
reminder of which data to collect so that the
management can at a later date evaluate how well the
care was delivered. And while with an EHR this
collection of information is easier and more
automated, there are still gaps on the usage of these
that limit the step by step tracking of clinical events.
One of our research aims is to investigate how we can
proactively support the clinical staff to adhere in real-
time to clinical pathways, with a greater focus on
delivery of care than on care delivery documentation.
We want to do this by going beyond the simple
digitization of the paper process.
Even though it is possible to identify processes in
healthcare, these are different from the ones that
could be found in an industrial manufacturing
environment (Mans et al., 2015). In the healthcare
environment, users are dealing with the care of
patients, who are not predictable machines with
predictable failure modes. Therefore enforcing a
process can be an extremely challenging task, let
alone having a system trying to follow the steps
making up that process. It is extremely difficult to
identify all the steps healthcare professionals follow
in a process performing retrospective data analysis
LeitÃ
ˇ
co A., Caffarel J. and Stretton E.
Translating a Clinical Workflow into a Computer-executable Model - User Needs Discovery, Challenges and Lessons Learned.
DOI: 10.5220/0006275005940600
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

since EHRs are rarely pre-programmed to support
clinical workflows in a structured manner. Therefore
any evidence we collect from these, represent point
measures of the process either indicating partial
activities in the process or post-documentation
information. Our research challenge was therefore to
understand how the users incorporate the clinical
processes in their routine, how they interact with the
systems, and how they could optimally be supported
with technology in the future to support them in real-
time to adhere to clinical pathways as they deliver
care to patients. There are no specific guidelines to
support extraction of requirements from end-users in
such situations, with most publications in the area
focusing on modelling of clinical processes, but not
giving insights onto the differences one might expect
across hospitals or recommendations how to translate
user specifications to technical requirements.
(Böckmann and Heiden, 2013; Dadam et al., 2000;
Latoszek-Berendsen et al. 2010; Staccini et al. 2001;
Oosterhout et al. 2005)
In this article we share some of our experiences
and methods used to go from an expressed wish from
users to actual user and technical requirements, using
the specific example of transforming workflows into
models that can then be used as part of an IT solution
to support workflow guidance.
In the specific study that we are using as example
we have confronted 4 different institutions with
different levels of understanding and complexity
regarding the same type of care delivery pathways
and associated workflows. We had a total of 51
participants in which 13 where physicians, 21 where
nurses (some with coordination roles within the
departments), 11 where quality staff and 6 had other
roles within the Hospitals. The Hospitals involved
have between 200 and 350 beds each and cover most
specialties inside the institution. Associated to this
they all have implemented an EHR but only one was
considered a paperless Hospital. Our expectation is
that models can allow us to create a unique translation
of realities, bringing together the workflows of the
different institutions. Therefore, we focus on the
specification of requirements for a technology
solution to assist the adherence to a Clinical Pathway
that involves multiple stakeholders, sometimes across
departments; and for a variety of care settings.
2 USER CONFRONTATION
2.1 Preparation Phase
Our team conducted user confrontations to validate
hypotheses, developed from literature reviews and
existing knowledge on the topic, on what users would
want in a workflow product. When researching how
to design these products to fit the needs of a specific
population of healthcare professionals, doing many
iterations of user confrontations is important. The
aim with the first user confrontations is to understand
what aspects of the problem cause the user the most
hindrance, and from there prioritize aspects of the
future product and create a research roadmap. For
each iteration after this, the scope of the
confrontations becomes more granular to specify
wishes for components of the proposed product.
Preparation for these confrontations consists of 3
phases: (i) identifying the activities to be done with
the clinicians and what type of professionals need to
be included, (ii) preparing legal and regulatory
documentation with our company and the hospitals
with which we will collaborate, (iii) contacting
hospitals and agreeing on an agenda.
(i) Identifying activities and participants: For
every round of user confrontations, we need to
identify:
a. What information we need to retrieve from
the activities (proving or disproving our
hypotheses) [more on this topic in the next
sub-section]
b. Who we should involve in the
confrontations in terms of professional
roles
Then, we plan activities that help us gather this
information. For each activity, we usually assign 2
researchers per activity, for 1-4 participants. This way
one person is able to take notes and another leads the
exercise. When performing user confrontations, a risk
that is run is that of collecting the opinion of too few
users and using these opinions to generalise to the
overall population of users. One way to address this,
if resources are limited, is to organise group activities
which enable multiple users’ viewpoints to be
collected in one session.
(ii) Legal and Regulatory: For conducting user
confrontations, there are legal and regulatory
agreements that need to be made on the
institutions involved, covering the interviewers
and the interviewees. It is important to keep this
in mind when planning the confrontations.
Translating the relevant documents such as
Non-Disclosure Agreements and Consent

Forms into the local language and allowing
time for reading and signing by the institution
could take several weeks. We strive to send the
Participation Information Letter and the
Informed Consent in advance of the visit, to
ensure the participants have had time to read it
and consider their involvement.
(iii) Preparing the agenda: When planning the
activities it is important to keep in mind time.
Healthcare professionals will typically have
30-45 minutes to spend in the activities. Lastly,
the agenda request must be made to the
hospital. In this request, there should be an
overview of the project, goals of the interviews,
and request for specific users for specific
amounts of time (for individual interviews and
group exercises).
2.2 Exercises Used by the Team
In this paper, we focus on the exercises carried out in
the first round of confrontation sessions with the
users. For this round, our main aim was to derive the
users’ main needs and challenges when it comes to
supporting the implementation of Clinical Pathways
in practice. As we were still in our project definition
phase, our scope was large: we wanted to learn more
about the topic from the users (general information on
how this is done in practice), from a variety of users
(from administrative to clinical and managerial staff)
and from all phases of Clinical Pathways (from the
creation of pathways, the use at the point of care to
the evaluation reporting). With this in mind, we
devised a number of activities:
Interviews to understand the users’ and
institutions’ realities and scope the landscape
of Clinical Pathways as it stands today in
clinical practice
A model building exercise, where we asked
the participants to build a pathway of their
choice and take us through their routine to help
us identify elements common to various
pathways as well as routine bottlenecks and
deviations from the set pathway
Confrontations of our work and assumptions:
- Review of a generic model we created, to
get some validation on the modelling of the
pathways
- Feedback on user interface designs for use
in supporting clinical and administrative
workflow to verify some assumptions we
made on information needs for clinical
users when it comes to needing to track
and care for a patient on a pathway
- Interactive activity enabling participants to
prioritise importance of pre-assumed
needs, as well as creation of unthought-of
needs
Questionnaires to derive quantitative results
related to the concepts explored with the users.
Whenever possible, we asked the users to walk us
through their way of working, showing us how they
interact with the clinical systems and indicating when
they would do this at various points of the care.
We had a variety of one to one and group settings.
Considerations when setting up group confrontations
are the following:
Size of group: 4-6 participants for 2 facilitators
is an appropriate size, larger groups may have
to be separated in sub-groups
Roles within group: hierarchy and personality
may influence the interaction dynamics within
a group, for overall opinion on a concept, we
prefer to separate the groups according to role;
mixing of roles can work well when
complimentary perspectives about different
aspects of a topic are sought
Facilitation skills: the facilitator of the activity
should direct the involvement of participants
when necessary, to ensure a fair representation
of all participants in the discussions
Discussion material: having material to discuss
(such as a concept video, screen designs, a
conceptual poster) can help the conversation
along, as a starting point or as a way to focus
the participants on the topic matter.
Overall, we derived a lot of useful information
from the users, which ranged from scoping a
landscape of realities and challenges from various
hospitals with varying levels of maturity when it
comes to implementing Clinical Pathways; all the
way to having a much clearer picture of the roadmap
we needed to create in order to meet the most pressing
needs of the users.
Some pitfalls we encountered were:
Broad scope and limited time meant that some
topics could not be deeply explored
Tight planning meant that not all exercises
could be conducted with all users
Questionnaire not specific enough to provide
significant added value on top of qualitative
results
Unexpected changes in times and personnel
available for participation in the activities.
Our recommendations include:
Be clear within the team on the objectives of
each exercise

Dry-run the activities with colleagues or proxy
users not involved in your project to ensure
instructions and questionnaires make sense
before finalisation
Double check translations (back translation if
possible) to ensure that the meaning is retained.
This might seem quite obvious but it is often
dismissed specially when using official
translators. It is important to make sure the
interpretation is the same for all readers no
matter the language.
Perform debriefing within the team as
frequently as possible, at least at start and end
of each day, and if possible, in between
activities especially in the first days to ensure
that the activities can be refined as the
interviews progress.
Finally, keep in mind that structured activities and
questionnaires are important to ensure focus is not
lost, but ability to improvise and follow the
participants’ train of thought in a semi-structured
interview format can often be invaluable to discover
user needs the team had not thought of or planned for.
2.3 Challenges of Interaction with the
Users
The first challenge of interacting with users on an
international level is communication. It is imperative
that the professionals that are participating in the
discovery activities, fully understand what is
presented so that your questions can be answered in
the end of the exercises.
To facilitate the understanding and better
communication we try to provide all the material and
conduct all the activities in the language of the users,
whenever possible.
Also, it is important to keep in mind that the realities
differ among institutions so the speech should be
adapted to the reality of each institution and
professional. You should always take into
consideration the following factors:
Technical resources. Not all institutions have
the same resources, such as, imaging machines,
beds or medications. This has a very high
impact on how the tasks are done, meaning that
the same step in a process can be executed in
different ways and sometimes even include a
third party institution who provides the
resource. A good model based system can help
not only to optimize the existing resources but
also to find the best workflow using the
resources available at each institution.
People. Not only is there variation in the
availability of staff, but also in the interaction
between different types of professionals among
the different institutions. As an example, in
some hospitals strict hierarchy may be the norm
(e.g. in one institution nurses may be
empowered to put a patient in a clinical
pathway whereas in another this may only be
done by a clinician). This has a big impact not
only on the identification of who should be
involved in a task but also on the attribution of
authority and permission for decision making.
This is so far the hardest factor that can affect
not only the way the exercises are done during
the user interactions but also can have a big
impact on how an IT solution will be used in
the institution. If you are looking to create a
solution which could be used in different
institutions it is important to identify all the
potential users, how they interact and who will
be the potential main users (which can include
different type of professionals)
Impact of geographical, organisational and
legislation factors on the level of maturity of
clinical processes. By association, institutions
that are involved with certification
organizations and medical societies usually
have very clear ways of working which are
based on best practices. This is also very
closely related to differences in implementation
of health services between different
institutions, regions and countries. In countries
where there are little or no public healthcare
institutions except for the primary care
facilities, most Hospitals and private
institutions will rely on certifications to
distinguish themselves from others. In the case
of countries where the Healthcare service is
very well managed by the government and
advances, chances are that standardization and
certification processes are stimulated if not
required by the government to guarantee the
minimum quality of services.
Knowledge. It is easy to assume that different
types of professionals have different levels of
knowledge. While that is true on a high level
and most people have greater knowledge on
their roles rather than on that of others, it is
good to not only explore their roles, but also
how they interact with and perceive the roles of
others in the organisation. When approaching
users from different institutions, cities or even
countries, we must take in consideration their
knowledge not only regarding technology (how
familiar are they with the latest technology and
how they use it in their work) but also on the
content level. As mentioned before, the
involvement of the professionals with the latest
news on best practices will also define how
prepared they are to understand the concept to
be discussed during the user interactions. And
as rewarding as it may be to involve Key
Opinion Leaders who are very much up to date
with state of the art and best practices in the
area you want to discuss, it can be even more
insightful to talk with users who have less or
little knowledge of the theoretical aspects so
that you can understand the real practical issues
the end-users are actually confronted with.
Nonetheless, it is good practice to assume the
users know very little about the topic, and be
prepared with a good but not restrictive
definition of the concept you want to present.
3 FILTERING KNOWLEDGE
INTO REQUIREMENTS
The main challenges we have had are:
How to transform information collected from
users into requirements usable by the technical
team?
How to ensure that the collected needs and
corresponding requirements are in some way
weighted to reflect the input from the variety of
users we interacted with?
To address the above, we employed a number of
methods, which included use of:
Raking/Rating systems where possible, e.g.
when confronting 3 user interface designs,
beyond asking for specific feedback, we also
asked the users to classify the designs from
their preferred one to least preferred one; for
the pre-assumed needs list, we asked the users
to rate each requirement as necessary, nice to
have, or not necessary
Quantitative data analysis wherever possible;
e.g., for the pre-assumed needs list, we
calculated a weighted average across the
groups and used this to rank the requirements
in order of importance, which gave an objective
perspective on the relative importance of the
rated requirements
Consensus technique whereby we analysed the
results of the interviews by first having a round
of insights extraction from our notes at an
individual interviewer level, before coming
together to share findings and debate if the
insights resonated with one or more
interviewees before including this as relevant
insights for our results.
Concerning Clinical Pathways, a main insight that
was drawn from our study with users is how to bridge
the technical viewpoint and the user viewpoint: there
are really two aspects to workflow modelling. One
level are the workflow elements needed for user
interaction to support them in their daily work; the
other level are those workflow elements which are
essential to the user to follow a Clinical Pathway, but
may not be relevant to model in technical terms,
either because it is not measurable or is difficult to
model, e.g. due to lack of evidence in the clinical IT
systems.
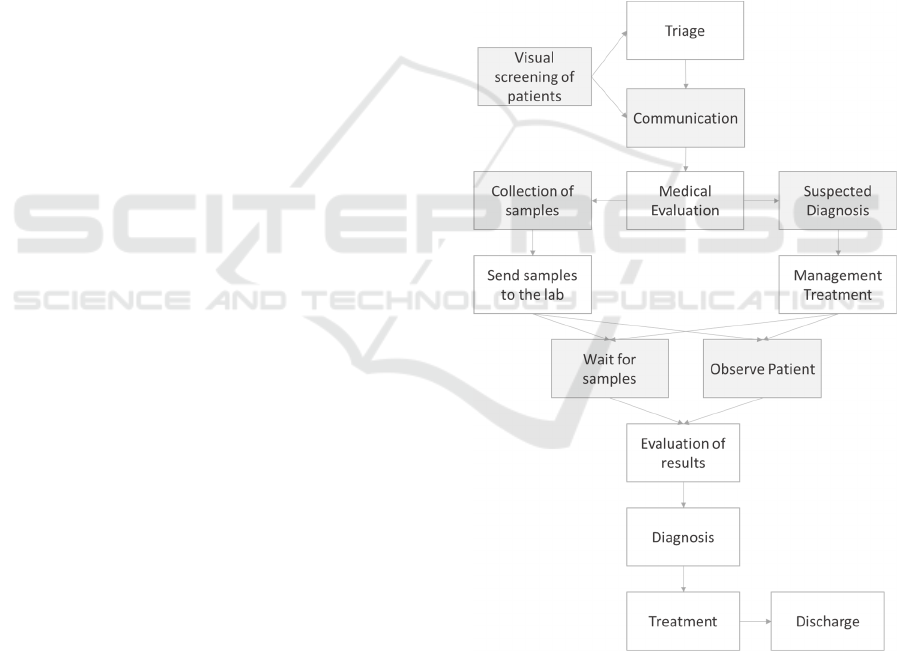
Figure 1: Simplified clinical process example as it would be
described by the user. The boxes in grey represent the
activities in the clinical process which are essential for the
users to carry out the process but only essential to the people
carrying out the task and not the model; or not captured in
the IT system because non-measurable or not included in
the IT documentation of the process.

Taking the example of a high level description of
a clinical process as described by a user such as the
one in Figure 1, we can identify 5 steps identified in
grey, of such type, e.g., the communication
interaction whereby the conversation process is more
important than the actual data exchanged. In the same
example we have the visual triage which is not
measurable since it is done mostly following the
professionals’ instinct and experience and it is not
associated with any record in the EMR; or activities
such as “collection of samples” which are not
captured in the EMR because so far, when the EMR
is used mainly for documentation of patient medical
data, there was no need to capture such process-
related information.
The same model can be translated into a machine
executable model, including only the steps that can be
found or recorded using the EMR, which would look
more like the model presented in Figure 2. Here we
can see loops appear in the place of a step by step
flow. While the user feels the need to represent every
step of the process as being unique, when mapping
these to the EMR the distinction loses relevance. For
example we can say that “Medical Evaluation” and
“Evaluation of results” are the same task since these
are represented by a new iteration of a clinical note in
the EMR.
Another big difference between a model described
by a clinical user and a technical model as the one of
Figure 2 is the detail and grouping of steps. We can,
for example, remove the “Suspected Diagnosis” step
described by the user as this is usually included only
as part of the clinical note. Also, steps that are in
distinct areas of the EMR and can be done in different
contexts outside the flow described by the user can be
represented as sub-processes. For this we have the
example of the “Diagnostic sub-process” or the
“Treatment sub-process” which can be done in a
different order or sequence than the one of Figure 1
when used in a different patient or clinical context.
Figure 2: Simplified clinical process example as it would be
used in the backend.
In the end we are left with only the steps which
can be detected from or triggered using specific
activities of the EMR. And while this might bring
some value in terms of process evaluation using the
EMR, it is not so useful if we are trying to support
and stimulate the users to follow guidelines or
processes using an abstract process model where the
same type of task can have different meanings and
relevance.
We believe that a model that reflects the habits
and routines of the professionals and not just the steps
/ recommendations of the protocols / guidelines /
pathways is the key to make a process support tool
operational and usable in clinical practice. That is, a
model which guides the users into doing the right
thing using more than just the steps that are recorded
in the EMR but also including those necessary for
their own routines. Such an ideal model based
solution would be the one that is capable of providing
the support for the human only tasks mentioned in
Figure 1, that usually have no place for representation
in the EMR (e.g. nurse calls the lab to check status of
sample analysis), even if they are not driving the
reasoning of the process. This support can be given
not only by making the association between the
modelling tools with Clinical Decision Support
Systems (CDS) but also organizational tools just like
communication, schedule assistance tools or others.
A severe limitation of modelling clinical processes
(whether prospectively or derived from process
mining) is the ability to derive representative models
despite some essential activities not being represented
in the event dataset.
Concerning those activities that are not possible to
model due to lack of evidence in the IT system,
these are essential to be aware of as this may imply:
an incorrect (incomplete) representation of the
process when performing process discovery.
Which consists in applying process mining
algorithms to an event log based on the
information from the Hospital’s EHR database,
to discover a process model. (van der Aalst, W.,
2016)
a necessary change to the IT system which may
have an impact on the workflow when trying to
derive a process model for real-time tracking of
process activities.
The latter has implications that go further than the
mere addition of a few requirements to the IT
solution: if the additional events cannot be captured
automatically, this will imply additional input from
the users affecting their workflow and potentially
adding burden to the overall process. If the
workflow is affected, this would also call for other

measures such as co-creation with the users and
change management leading up to and during
introduction of the technology to ensure acceptance
and good uptake of the solution.
4 CONCLUSIONS
Deriving clinical processes based on data available in
EHRs is a challenge for a number of reasons: different
hospitals are likely to implement similar processes in
different ways due to different resources available
and local constraints; not all process activities may be
directly extractable from the data, due to lack of
documentation or impossibility to capture in a
structured format; any additional process-related data
which needs to be acquired may be seen as an
additional burden on the users and may impede the
actual process we are trying to support. When
extracting knowledge from users to determine
relevant events from data or to derive process models,
one must be aware of the different realities of each
setting and user’s role, and try to capture the overall
process by approaching the various stakeholders that
often work together to make the entire clinical
process a reality.
It is really important to find a balance between the
tasks that need to be represented and shown to the
user and the tasks that can be automated relieving
burden from the user. For a good workflow support
system we do not necessarily need to present all the
steps of the process to the user nor represent in the
model all the intermediate steps that are taken by the
user. More than a good model, you will need extra
support systems that can fill the gaps and fix the
bottlenecks of the workflows.
ACKNOWLEDGEMENTS
We would like to thank the participants in our
research study for time and valuable input, as well as
our colleagues from our Research project for making
this work possible.
REFERENCES
Ash, J., Bates, D., 2004. Position Paper. “Factors and
Forces Affecting EHR System Adoption: Report of a
2004 ACMI Discussion” Journal of the American
Medical Informatics Association 2005; 12:8–12.doi:
10.1197/jamia.M1684.
Böckmann, B., Heiden, K. 2013. “Extracting and
Transforming Clinical Guidelines into Pathway Models
for Different Hospital Information Systems.” Health
Information Science and Systems
1:13.doi:10.1186/2047-2501-1-13.
Coiera, E., 2000. “When Conversation Is Better Than
Computation.” Journal of the American Medical
Informatics Association 7 (3): 277.
doi:10.1136/jamia.2000.0070277.
Dadam, P., Manfred R., Klaus K. 2000. “Clinical
Workflows — The Killer Application for Process-
Oriented Information Systems?” In BIS 2000: 4th
International Conference on Business Information
Systems, Poznań, Poland, 12–13 April 2000, edited by
Witold Abramowicz and Maria E. Orlowska, 36–59.
London: Springer London. http://dx.doi.org/
10.1007/978-1-4471-0761-3_3.
Jamoom, E. W., Heisey-Grove, D., Yang, N., Scanlon, P.,
2016. “Physician Opinions about EHR Use by EHR
Experience and by Whether the Practice had optimized
its EHR Use” Journal of Health & Medical Informatics
7 (4) OMICS International., ISSN: 2157-7420.
Latoszek-Berendsen, A., Tange, H., van den Herik, H.,
Hasman. A. 2010. “From Clinical Practice Guidelines
to Computer-Interpretable Guidelines. A Literature
Overview.” Methods of Information in Medicine 49 (6):
550–70. doi:10.3414/ME10-01-0056.
Mans, R., van der Aalst, W., Vanwersch, R., 2015. Process
Mining in Healthcare: Evaluating and Exploiting
Operational Healthcare Processes; Springer.
Ologeanu-Taddei, R., Morquin, D., Domingo, H., &
Bourret, R., 2015. “Understanding the acceptance
factors of an Hospital Information System: evidence
from a French University Hospital.” AMIA Annual
Symposium Proceedings, 2015, 1001–1007.
Oosterhout, E., Talmon, J., Clercq, P., Schouten, H., Tange,
H., Hasman, A., 2005. “Three-Layer Model for the
Design of a Protocol Support System.” MIE 2003 74
(2–4): 101–10. doi:10.1016/j.ijmedinf.2004.04.023.
Staccini, P., Joubert, M., Quaranta, J., Fieschi, D., Fieschi.
M., 2001. “Modelling Health Care Processes for
Eliciting User Requirements: A Way to Link a Quality
Paradigm and Clinical Information System Design.”
International Journal of Medical Informatics 64 (2–3):
129–42. doi:10.1016/S1386-5056(01)00203-9.
van der Aalst, W., 2016. “Process Mining: Data Science in
Action”, Springer.
