
In-vitro Modeling of Electrode-tissue Parameters
Maran Ma and Timothy E. Kennedy
Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montréal, Canada
1 OBJECTIVES
The long-term causes underlying the failure of neural
recording electrodes is an active question in the
community of neural implant users and developers. It
is known that a variety of phenomena contribute to
signal degradation, but to engineer better devices, the
impact of each factor must be quantified and
prioritized. Excluding modes of general implant
failure such as connectors and meningeal responses
(Barrese, et al., 2013), the causes of gradual loss of
signal fidelity on individual electrodes include: 1)
insulation breakdown (Prasad, et al., 2014), 2)
biofouling (Malaga, et al., 2015), 3) glial
ensheathment (Polikov, et al., 2005), and 4) neuronal
death (Biran, et al., 2005). It is difficult to identify the
dominating problem based on existing literature,
which offer different conclusions (Prasad, et al.,
2014; Malaga, et al., 2015).
One challenge to teasing these factors apart is that
they occur simultaneously in-vivo. Another is that
impedance, the primary method of monitoring
electrode status, is affected by multiple factors. Not
surprisingly the correlation between impedance and
signal quality has been reported to be weak in
longterm studies of intracortical arrays, and changes
in strength and direction during different time periods
post implantation (Barrese, et al., 2013).
The purpose of this project is to build a simple and
cost effective in-vitro setup to model each
phenomenon individually, and quantify its impact on
both impedance spectra and electrophysiological
recording quality.
2 METHODS
2.1 Hardware Components
A custom adapter board was built to interface with
commercially available multi-electrode array (MEA)
plates (single well plate, Axion Biosystems).
Electrical contact is made via Z-axis elastomer, and
secured in place with a 3D printed housing. The
adapter board was laid out such that electrode
positions in the MEA mapped to a corresponding
field of headers.
Figure 1: A. Adapter board for interfacing with MEA plate.
B. MEA plate seated in adapter board, two electrode sites
connected to impedance converter board.
A single-chip impedance converter / network
analyser (AD5933, Analog Devices) is used to
perform electrochemical impedance spectroscopy.
The device was modified for optimal measurement of
1Khz -10Khz range by adding a 4Mhz oscillator.
A generic data acquisition module with built-in
instrumentation amplifier (DAQPad-6259, National
Instruments) and custom LabVIEW programs were
setup for electrophysiological recordings.
2.2 Biological Components
Culture of rat embryonic (E18) cortical neurons and
postnatal (P1) astrocytes and microglia will be used
to model neural and glial-scar tissue in the MEA
plate. Inflammation inducing factors (such as TGFβ1)
will be added to convert glial cells to a state of
reactive gliosis. The HEK293 cell-line is used to
generate reference data on effects of cell density.
2.3 Test Conditions
The four conditions will be modelled as follows:
• Biofouling: incubate MEA plate with 100%
fetal bovine serum (FBS).
Ma M. and Kennedy T.
In-vitro Modeling of Electrode-tissue Parameters.
In NEUROTECHNIX 2017 - Extended Abstracts (NEUROTECHNIX 2017), pages 12-13
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
• Glial ensheathment: seed glial cells at
various densities on top of a neural culture.
• Neuronal death: increase distance between
MEA surface and neural culture with
hydrogel coating or a dense glial layer.
• Insulation breakdown: natural degradation
of the MEA plate (insulation layer begins to
detach with repeated usage/sterilization).
3 RESULTS
Preliminary testing of the in-vitro setup showed
sufficient sensitivity and consistency in impedance
measurements to proceed to electrophysiological
experiments.
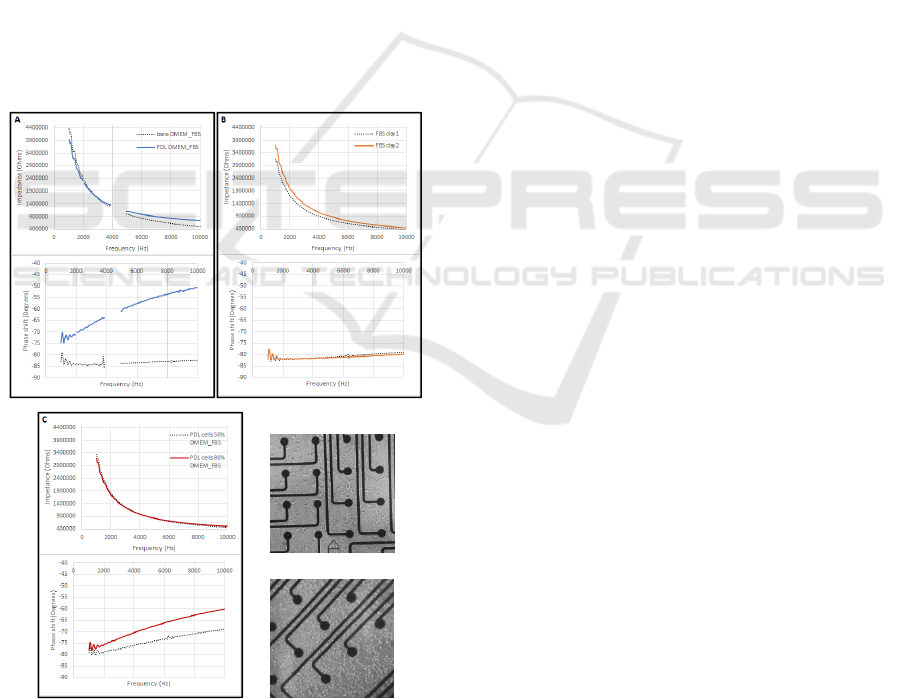
Differences in spectra were observed after
modifications to the MEA surface: before and after
PDL coating (Figure 2A), after one day of FBS
incubation (Figure 2B), and at HEK293 cell densities
of ~50% and ~80% confluence (Figure 2C).
Figure 2: A. Before and after PDL coating, measured in
DMEM solution with 10% FBS (typical for HEK293 cell
culturing). B. After one day of incubation in serum (100%
FBS). C. HEK293 cell culture at different densities.
4 DISCUSSION
This system for in-vitro impedance testing and
electrophysiology combines relevant aspects of a
neural electrode to tissue interface in a simple setup.
Commercial disposable MEA dishes are affordable,
well fabricated to work with in-vitro methods, and
transparent for convenient imaging. The custom
adapter board enables flexible access to MEA sites
for connecting to any instrumentation. The single-
chip impedance converter and general purpose data
acquisition box provide low-cost and programmable
options for interrogating the MEA plate.
Preliminary impedance data show promise that
different electrode-tissue conditions yield different
spectra. This may offer a means to reconcile
observations in the literature regarding impedance
and signal quality trends. A potential further
application of this system is screening of electrode
coating materials for enhancing biocompatibility.
REFERENCES
Barrese, J. C. et al., 2013. Failure mode analysis of silicon-
based intracortical microelectrode arrays in non-human
primates. Journal of Neural Engineering, 10(6).
Biran, R., Martin, D. C. & Tresco, P. A., 2005. Neuronal
cell loss accompanies the brain tissue response to
chronically implanted silicon microelectrode arrays.
Experimental Neurology, 195(1).
Malaga, K. A. et al., 2015. Data-driven model comparing
the effects of glial scarring and interface interactions on
chronic neural recordings in non-human primates.
Journal of Neural Engineering, 13(1).
Polikov, V. S., Tresco, P. A. & Reichert, W. M., 2005.
Response of brain tissue to chronically implanted
neural electrodes. Journal of Neuroscience Methods,
148(1).
Prasad, A. et al., 2014. Abiotic-biotic characterization of
Pt/Ir microelectrode arrays in chronic implants.
Frontiers in Neuroengineering, 7(2).
~50% confluence
~80% confluence
