
Wheelchair Exercise Monitor Development Platform
An Application for Wireless EMG Sensors
Amit Pal
1
, Kevin Monsalvo
1
, James Sunthonlap
2
, Paolo Arguelles
1
, Aldo Adame
1
, Jackson Tu
1
,
Ellie Tjara
1
, James Velasco
1
, Terrence Sarmiento
1
, Roxanna Pebdani
3
, Christine Dy
4
,
Stefan Keslacy
4
, Ray de Leon
4
and Deborah Won
1
1
Department of Electrical and Computer Engineering, California State University, Los Angeles, CA, U.S.A.
2
Department of Computer Science, California State University, Los Angeles, CA, U.S.A.
3
Department of Special Education, California State University, Los Angeles, CA, U.S.A.
4
Department of Kinesiology, California State University, Los Angeles, CA, U.S.A.
Keywords:
EMG, Wireless Sensor, Exercise Monitor, Individuals Who Use Wheelchairs, IMU, Heart Rate Monitor,
Wearable Sensors, Sensor Integration, Fitness Metric, Fitness Tracking Mobile App.
Abstract:
We present here a novel application for wireless EMG sensors. To combat the physical inactivity which has
tended toward cardiovascular disease in individuals who use wheelchairs, we have developed a monitoring
system to encourage these individuals to exercise. Wireless sensors are used to monitor kinematic or physi-
ological metrics, which inform the user of their activity levels during exercise and to track progress of their
fitness levels over time. In particular, a new completely wireless, wearable EMG sensor (Dynofit, Inc., TX) is
integrated with accelerometer and heart rate sensor data to monitor energy expenditure. The sensors communi-
cate with a custom designed mobile app which facilitates exercise at home, with the aim of helping individuals
who use a wheelchair to overcome what are commonly hindrances to exercising.
1 CARDIOVASCULAR FITNESS
IN WHEELCHAIR USERS
Individuals who use wheelchairs have an increased
risk of cardiovascular disease (Selassie et al., 2013;
Garshick, 2005). To address this growing health is-
sue, and to support preventative measures for car-
diovascular co-morbidity, we are developing a sys-
tem which would promote and facilitate exercise for
wheelchair users. (Abel et al., 2008; Blair, 1999).
A widely accepted recommendation for reducing car-
diovascular disease risk has been to increase average
daily energy expenditure by 300-350 kilocalories (RS
et al., 1993).
Thus, it has been important to the research com-
munity to determine what forms and amounts of ex-
ercise should be prescribed to reach this fitness goal.
However, those who use wheelchairs face many barri-
ers to exercising the right way and right amount, many
tied to the challenge of getting to specialized gyms or
wellness centers.
To encourage and support wheelchair-dependent
persons in meeting these recommendations for caloric
expenditure, we are developing an in-home exercise
program, so that their workouts do not depend on
having access to expensive and/or large equipment,
and/or to therapists with whom scheduling or trans-
portation also disincentivizes exercising. In keeping
with the current trend of fitness trackers, we are de-
veloping a system to track a measure of fitness to mo-
tivate individuals to exercise and be encouraged by
progress in fitness and/or activity levels. As energy
expenditure has been agreed upon as the most infor-
mative metric of cardiovascular fitness (Abel et al.,
2003; Ainsworth et al., 1993), at the crux of this
in-home exercise monitoring system is the ability to
monitor energy expenditure continuously at home,
during exercise.
2 MEASURING ENERGY
CONSUMPTION
Currently available or researched activity monitors
and fitness trackers for mobility impaired individuals
predominantly rely on acceleration, heart rate, or a
Pal, A., Monsalvo, K., Sunthonlap, J., Arguelles, P., Adame, A., Tu, J., Tjara, E., Velasco, J., Sarmiento, T., Pebdani, R., Dy, C., Keslacy, S., Leon, R. and Won, D.
Wheelchair Exercise Monitor Development Platform - An Application for Wireless EMG Sensors.
DOI: 10.5220/0006610000670073
In Proceedings of the 7th International Conference on Sensor Networks (SENSORNETS 2018), pages 67-73
ISBN: 978-989-758-284-4
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
67

combination of the two. Clinically relevant outcomes
for wheelchair users, such as amount of movement,
distance travelled, strength of maximum voluntary
contractions, and wheelchair propulsion have been ef-
fectively quantified with various sensing mechanisms,
the most common being the accelerometer or a reed
switch-based data logger(1). Accelerometry and the
data logger provide a reasonable measure of move-
ment and wheelchair propulsion (2), but motion de-
tectors are prone to false positives, as in the case of
an accelerometer-based step counter, which would de-
tect shaking of the device up and down and mistake
such motion as exercise. EMG is also better suited to
tracking compliance with prescribed exercises, since
the pattern of activation across multiple muscles can
be monitored. Furthermore, in contrast to accelerom-
eters, EMG may not only be used to monitor the con-
tractions of individual muscles, but also to capture
muscle activations during isometric contractions.
Energy expenditure is consistently relied upon
to measure and predict cardiovascular disease risk
(Sawka et al., 1980). However, measuring energy
expenditure directly through whole body calorime-
try or through oxygen uptake (VO
2
) measurements
requires expensive and/or impractical equipment and
facilities. Heart rate can be used to fairly accurately
compute energy expenditure (?). However, heart rate
is difficult to obtain accurately during exercise, and
in particular, for spinal cord injury patients. Auto-
nomic dysfunction is common in spinal cord injured
patients (Krassioukov et al., 2008), and we have ob-
served anomolous heart rate recordings in spinal cord
injury subjects during exercise.
3 DREAM EXERCISE
MONITORING SYSTEM
DESIGN
The DREAM (Disability, Rehabilitation, and Engi-
neering Access for Minorities) exercise monitoring
system is designed to provide feedback which moti-
vates the user to improve his/her cardiovascular fit-
ness through exercise. The main system components
are shown in Fig. 1.
Muscle activation, heart rate, and endpoint accel-
eration are measured in real-time from wireless sen-
sors, which include Flexdot EMG sensors (Dynofit,
Carrollton, TX), a Wahoo Tikr heart rate moni-
tor (Wahoo Fitness, Atlanta, GA), and a custom-
packaged inertial monitoring unit. The DREAM app
allows users to set HR and activity target zones, con-
tinuously monitor their activity levels, and track the
Figure 1: System components of the DREAM exercise
monitor: A) Dynofit’s Flexdot wireless EMG sensor; B)
the DREAM mobile app; C) the custom packaged wireless
IMU board; D) Mio Global’s Alpha 2 heart rate monitor
(Mio Global, Vancouver, British Columbia).
Table 1: Performance specifications.
EMG HR IMU
output
metric
integrated
envelope
heart rate wrist ac-
celeration
units % of max.
isometric
beats per
minute
m/s
2
sampling
rate (sam-
ples/sec)
60 1 4
input
range
±300µV 0-200bpm ±8g
resolution 440µV
(12-bit)
1bpm 940µg
(14-bit)
current
con-
sumption
(active
mode)
3mA <1mA 12.5mA
battery
life (hrs of
active use)
73hrs 300hrs 41 hrs
monitored metrics over time. The performance speci-
fications for the DREAM monitoring device are given
in Table 1
The fitness-relevant physiolgical metrics are mon-
itored and wirelessly transmitted to a smart phone app
which depicts the metrics in an easy-to-read and mo-
tivating way. Fig. 3 illustrates the screen users will
see when exercising.
This cloud-based multi-user functionality and web
portal have yet to be implemented.
Here we describe the unique sensing components
of our design in greater detail.
SENSORNETS 2018 - 7th International Conference on Sensor Networks
68
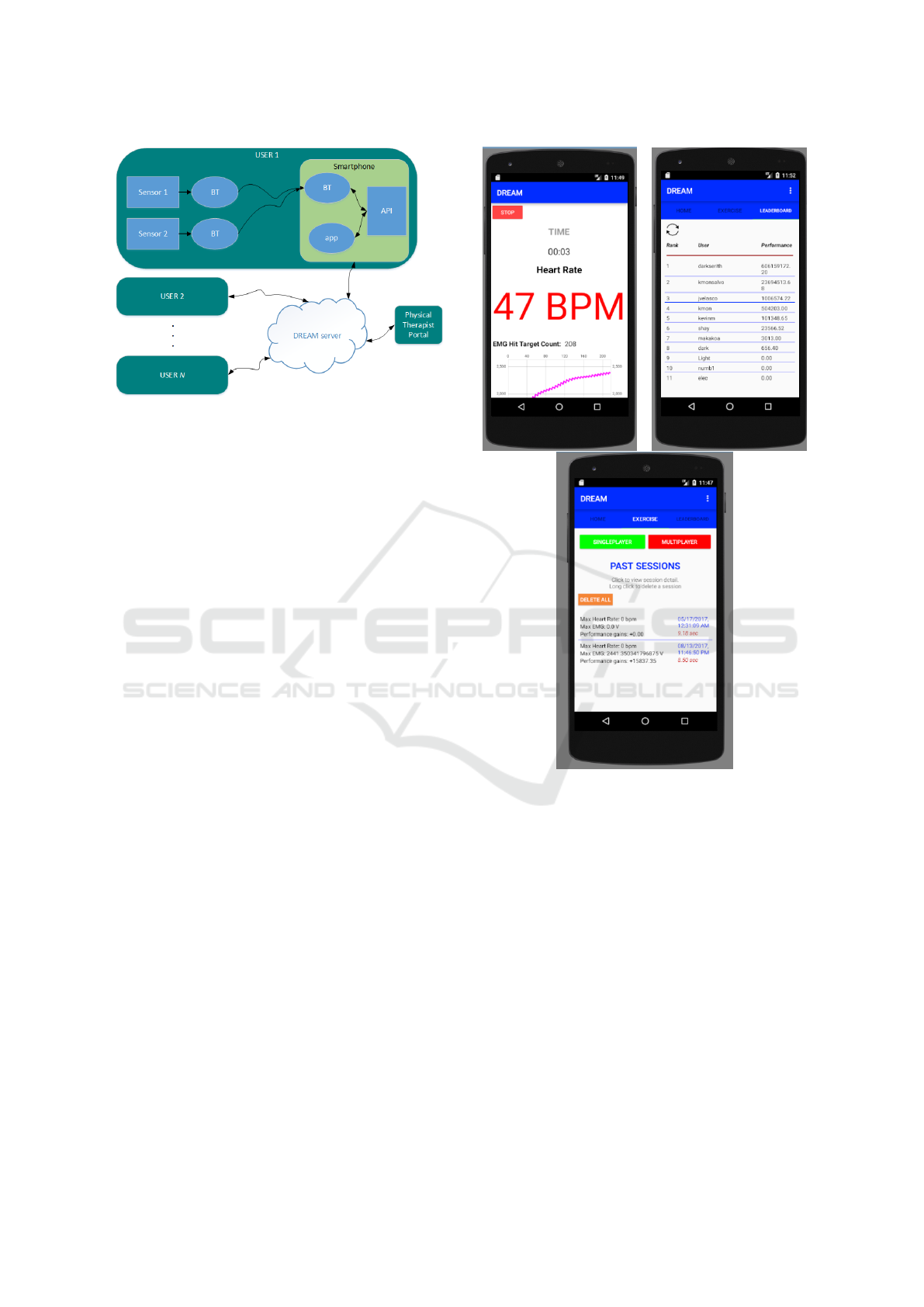
Figure 2: Schematic system diagram of a next generation
DREAM exercise monitor.
3.1 EMG Sensors
EMG is acquired by commercial sensors developed
by Dynofit to allow for completely wireless EMG ac-
quisition tailored for mobile applications. Other ven-
dors manufacture wireless EMG sensors, but these
sensors are more well suited for research applications,
as they require proprietary hardware (a base station)
and software. In contrast, the Dynofit Flexdots com-
municate via Bluetooth Low Energy (BLE), and all
the hardware needed to perform wireless communica-
tion with a smartphone is contained within the wear-
able sensing unit. The sole requirement to acquire
data from the Flexdot is the capability of the receiver
to communicate via BLE.
The Flexdot module is fully packaged into a plas-
tic housing with snap female connectors for the elec-
trodes. Disposable electrolyte gel-filled electrodes are
snapped into the electrode contact terminals. Inside
the plastic housing is a custom board which provides
signal conditioning and A/D conversion, a BLE trans-
mitter, and a lithium coin cell battery.
3.2 Wearable IMU Module
During this development phase of the wheelchair ex-
ercise monitoring system, in order to determine the
most suitable fitness metric, commonly used metrics
of activity will be acquired and compared to novel
metrics which incorporate or even rely centrally on
EMG measurements. Activity monitors most com-
monly measure heart rate and acceleration. An off-
the-shelf popular heart rate monitor, the Wahoo Tikr
is able to be used in the DREAM development plat-
form because the Tikr transmits data via the ANT+
wireless protocol. Thus, the DREAM app can com-
municate directly with the Tikr without need for any
Figure 3: Screenshots of two of the main DREAM app
screens: 1) activity metrics page displayed in real-time dur-
ing an exercise session; 2) the “Leaderboard” showing the
ranking of DREAM app users, ranked according to a fitness
metric; 3) a log of stored data from past exercise sessions,
all accessible from the DREAM cloud server.
proprietary information. However, the top-of-the-line
accelerometers that are most often used in activity
monitoring in the literature pose the same issues that
the wireless EMG did; namely, they require connec-
tion to a PC and proprietary software. From pre-
liminary data, we had already seen that accelerom-
etry alone would not provide sufficiently accurate
feedback about energy consumption. Therefore, we
custom built a wearable BLE-based wireless inertial
monitoring unit module.
The system components and IMU module design
are provided in the board schematic in Fig. 5. This
custom PCB board consists of an IMU IC, BLE-
Wheelchair Exercise Monitor Development Platform - An Application for Wireless EMG Sensors
69
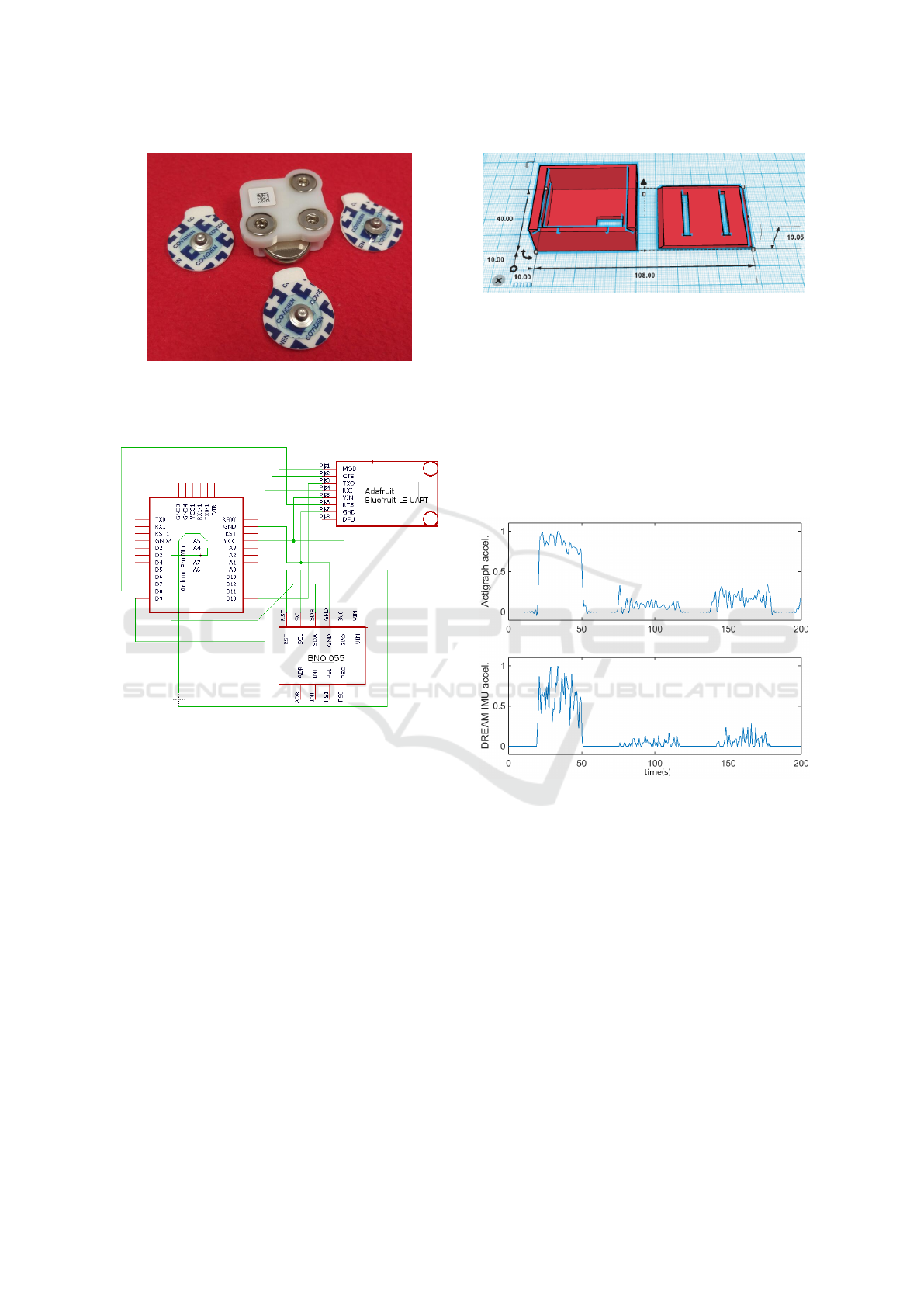
Figure 4: Flexdot components: the plastic housing for the
signal processing circuitry, with 3 female snap connections,
a 3V-coin cell battery, and the disposable snap electrodes
(Medtronic Covidien PLC, Minneapolis, MN).
Figure 5: Circuit schematic of IMU module.
UART wireless adapter, and Arduino microcontroller
board. The BNO055 is an intelligent 9-axis orienta-
tion MEMS sensor with a UART interface. We use
only the accelerometry measurements. In order for
our mobile app to acquire acceleration data wirelessly
from the BNO055, we interface the BNO055 with
a BLE transmitter on board the Bluefruit LE-UART
Friend (Adafruit Industries). The Bluefruit board is
a UART wireless adapter that establishes serial com-
munication with the BNO055 and then transmits this
data through the BLE transmitter to the mobile smart
phone. The ATMega 328P microcontroller, on board
an Arduino Pro Mini, controls the UART communi-
cation between the BNO055 and Bluefruit board.
This IMU module is powered by two 3.7V Li-ion
Polymer batteries in series, and fits in a custom hous-
ing unit that can be worn via velcro strap around the
wrist. The Solid Works CAD drawing for the housing
unit is provided in Fig. 6. The package has slots for
the velcro strap and space for a power switch. The
housing is approximately 4cm x 4cm x 2cm.
Figure 6: CAD design for the IMU module housing unit,
3d-printed in ABS plastic. Dimensions are provided in mm.
Magnitude of 3D acceleration recorded during
wheelchair pushes, tricep extensions, and lat rows
for approximately 30 second bouts, with 30-second
rest intervals, is shown in Fig. 7. The DREAM IMU
module appears to record similar magnitude as Acti-
graph, but the Actigraph shows less high frequency
noise. We plan to implement low-pass filtering in the
DREAM IMU module to obtain acceleration wave-
forms which even more closely match Actigraph’s.
Figure 7: Endpoint acceleration magnitude acquired by
Actigraph sensor vs. DREAM IMU module.
4 PERFORMANCE OF EMG
SENSOR
Before Dynofit’s existence, a number of commercial
wireless EMG sensors existed on the market. These
are generally high performance sensors with excel-
lent noise cancellation, motion artifact suppression,
and overall signal to noise ratio. However, to the au-
thors’ knowledge, all of these wireless EMG sensors
require a receiver base station and a PC workstation
with proprietary software for data acquisition. We se-
lected the Dynofit Flexdot for EMG sensing because
of its standalone capability; i.e., the Flexdot requires
no base station but transmits data wirelessly through
SENSORNETS 2018 - 7th International Conference on Sensor Networks
70
Bluetooth Low Energy (BLE) without requiring any
proprietary software. These features make it well
suited for our in-home exercise application, whereas
the other high performance EMG systems are better
suited for research applications or patient assessment
in a clinical setting but not practical for our applica-
tion of in-home exercise monitoring.
While the Dynofit Flexdot sensors were more
practical and were the only sufficiently practical EMG
wireless sensors of which we were aware, we also
wanted to test the performance of the EMG sensors
relative to one of the top-of-the-line wireless EMG
commercial systems. We selected the Delsys Trigno
to which to compare the Flexdot.
A Flexdot was adhered to the muscle belly of the
right bicep; a Trigno sensor was adhered to the muscle
belly as well, adjacent to the Flexdot. EMG was ac-
quired from both systems for 60 seconds during bicep
curl exercises and isometric contractions. The EMG
envelope was obtained by full-wave rectifying the raw
EMG, and then applying a moving average filter with
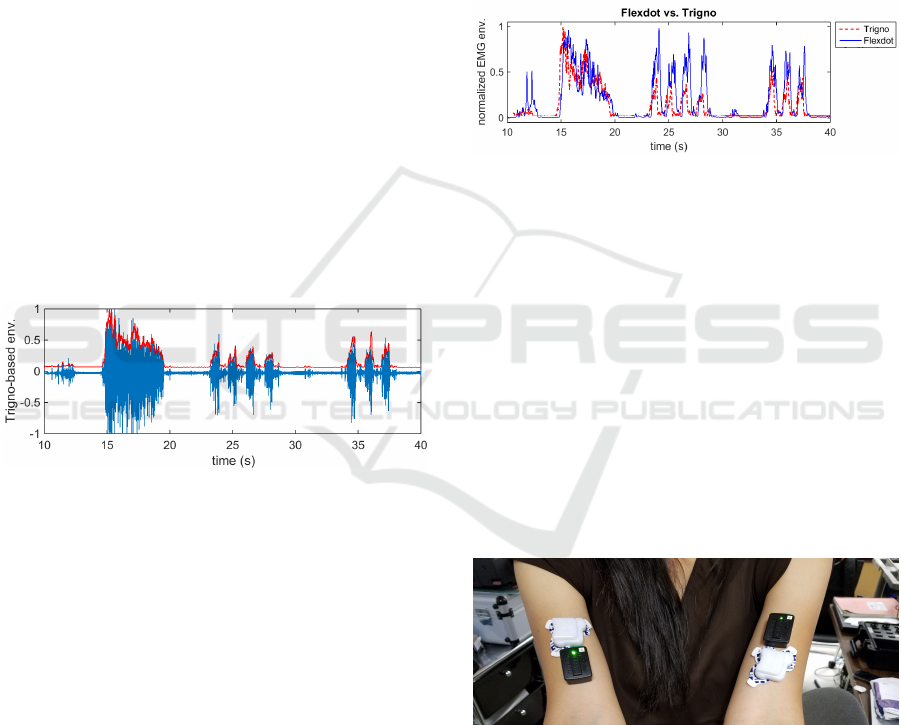
a rectangular window of 100ms. Figure 8 illustrates
that the envelope was appropriately obtained from the
raw EMG. Both envelope amplitudes were normal-
ized to range between 0 and 1.
Figure 8: EMG envelope superimposed on the raw EMG
acquired from the Trigno sensor.
The envelope obtained from each of the sensors
were compared, as shown in Fig. 4. While the Flexdot
captured each muscle activation and showed temporal
accuracy, the amplitude of muscle activation some-
times exceeded that of the Delsys Trigno in this ap-
plication. Factors contributing to the amplitude dif-
ferences may include the differences in the location of
the sensors on a single muscle, spacing of electrodes
on the devices, size and the conductivity of the pads
used to adhere the device to the skin above the mus-
cle. These factors were not likely to explain the dif-
ferences, however, given that these amplitude changes
were observed for a given subject within the same
recording session. What appeared to be more likely
the case is that adaptive filtering is being applied to
maximize use of the dynamic range on the amplitude
scale, such that the normalization of relative ampli-
tude is adjusted over time. Switching to non-adaptive
normalization is simply a matter of adjusting the post-
processing in firmware.
In order to quantify a direct comparison between
the Flexdot-based and Trigno-based EMG activity,
the Flexdot data was first upsampled, since the for-
mer was acquired at 64Hz , and the latter at 2000Hz.
We then computed the RMS difference between the
two normalized envelopes, and obtained an RMS er-
ror of 0.27. Since the amplitudes are normalized, we
represent the RMS difference as 27% of the amplitude
range. As indicated above, this difference can be at-
tributed to the post-processing methods implemented
in firmware.
Figure 9: Comparison of EMG envelope acquired from the
Dynofit Flexdot by the DREAM app and the EMG envelope
obtained from Delsys Trigno.
To help confirm that the differences were more
likely due to differences in post-processing schema
than to physical characteristics, such as size and lo-
cation of the electrodes, we conducted another test.
This time, the subject performed bicep curls with elas-
tic arm bands (TheraBand, Akron, OH) at 3 levels of
increasing resistance. Recordings were taken from a
Trigno sensor placed on the left arm slightly proximal
to the center of the muscle belly, and a Flexdot sensor
placed just distal to the Trigno sensor, such that they
both overlapped with the center of the muscle belly.
On the right arm, we had the converse placement of
sensors, as seen in Fig. 10.
Figure 10: Placement of wireless EMG sensors. Left arm:
Trigno sensor placed more proximally, Flexdot sensor more
distally. Right arm: Flexdot sensor placed more proximally,
Trigno more distally.
Five bicep curls were conducted at each resistance
level. To increase the resistance level, we merely
shortened the theraband to fixed lengths (of 80, 60,
and 40 cm). The resulting EMG envelopes are shown
in Fig. 11 for the left arm, and Fig. 12 for the right
arm.
Wheelchair Exercise Monitor Development Platform - An Application for Wireless EMG Sensors
71
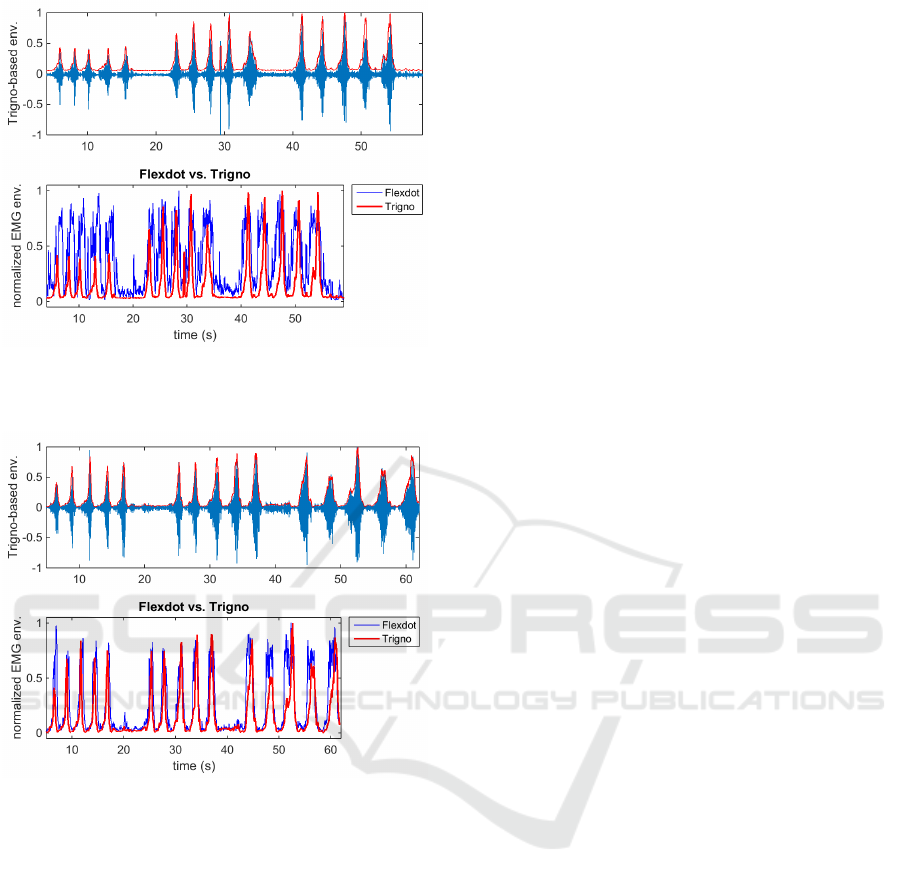
Figure 11: Comparison of Trigno-derived vs. Flexdot-
derived EMG envelopes (from left arm) with Trigno sensor
more proximal, Flexdot more distal.
Figure 12: Comparison of Trigno-derived vs. Flexdot-
derived EMG envelopes (from right arm) with Trigno sen-
sor more distal, Flexdot more proximal.
The increasing amplitude of the Trigno envelope
corresponds with the increasing resistance of the arm
bands. It is possible that the Flexdot envelope has an
adaptive gain that was designed to maximize use of
the dynamic range at all times. The Flexdot performs
very well in terms of temporal resolution, which in
our application is critical. The location of the sensor
does have some influence on the EMG amplitudes,
but the main cause of the difference in amplitudes ap-
pears to lie within the post-processing in the sensors’
firmware.
How accurate the amplitude needs to be in order to
provide a motivating, reliable fitness metric to poten-
tial DREAM app users has yet to be researched. Ad-
justment of the EMG envelope amplitude is expected
to require a straightforward adjustment of low-pass
filtering parameters applied to compute the envelope.
5 CONCLUSIONS
Wireless sensing capabilities of EMG, heart rate,
and accelerometry have been integrated into a single
mobile app-based exercise monitoring system. The
DREAM system is being designed to help motivate
individuals who use wheelchairs to improve their car-
diovascular fitness through exercise. We have pre-
sented the design and implementation of a first proto-
type which will enable us to research the most appro-
priate fitness metric on which to provide motivating
feedback to the users. In particular, we highlight the
selection and use of a new standalone wireless EMG
sensor which performs with signal quality compara-
ble to high-end wireless EMG sensors on the market,
with the added benefits of low cost and practicality
in an in-home exercise application that is predicted to
help advance rehabilitation therapy.
ACKNOWLEDGEMENTS
This work was funded by an NIDILRR Field Initiated
Projects Program (Grant #90IFST0001-01-00). The
authors would like to gratefully acknowledge Dynofit
founders and Flexdot developers Maria Schneider,
Edward Rosten, Alex Macdonell, Rohan Loveland,
and Mary Cooley who have generously offered their
time to help us learn more about their product.
We would also like to gratefully acknowledge Joel
Ramirez, Lloyd Ruiz, Lisa Le, and Isali Win who
have offered their time and effort to help in various
ways to carry out testing.
REFERENCES
Abel, T., Kr
¨
oner, M., Rojas, V. S., Peters, C., Klose, C., and
Platen, P. (2003). Energy expenditure in wheelchair
racing and handbiking-a basis for prevention of car-
diovascular diseases in those with disabilities. Euro-
pean Journal of Cardiovascular Prevention & Reha-
bilitation, 10(5):371–376.
Abel, T., Platen, P., Vega, S. R., Schneider, S., and Str
¨
uder,
H. (2008). Energy expenditure in ball games for
wheelchair users. Spinal Cord, 46(12):785.
Ainsworth, B., Haskell, W., Leon, A., Jacobs, D. J., Mon-
toye, H., and Sallis, J. (1993). Compendium of phys-
ical activities: classification of energy costs of. Med
Sci Sports Exerc, 25:71–80.
Blair, S. (1999). Effects of physical inactivity and obesity
on morbidity and mortality: current evidence and re-
search issues. Medicine and science in sports and ex-
ercise, 31(11 suppl):S646–S662.
SENSORNETS 2018 - 7th International Conference on Sensor Networks
72

Garshick, E. e. a. (2005). A prospective assessment of
mortality in chronic spinal cord injury. Spinal Cord,
43:408–416.
Krassioukov, A., Karlsson, A.-K., Wecht, J. M., Wuermser,
L.-A., Mathias, C. J., and Marino, R. J. (2008). As-
sessment of autonomic dysfunction following spinal
cord injury: Rationale for additions to international
standards for neurological assessment. Journal of
Rehabilitation Research & Development, 44(1):103–
112.
RS, P. J., RT, H., AL, W., IM, L., DL, J., and JB, K.
(1993). The association of changes in physical-
activity level and otherlifestyle characteristics with
mortality among men. N Engl J Med, 328:538–545.
Sawka, M. N., Glaser, R. M., Wilde, S. W., and von Luhrte,
T. C. (1980). Metabolic and circulatory responses to
wheelchair and arm crank exercise. Journal of Applied
Physiology, 49(5):784–788.
Selassie, A., Snipe, L., Focht, K. L., and Welldaregay, W.
(2013). Baseline prevalence of heart diseases, hyper-
tension, diabetes, and obesity in persons with acute
traumatic spinal cord injury: potential threats in the
recovery trajectory. Top. Spinal Cord Inj. Rehabil.,
19:172–182.
Wheelchair Exercise Monitor Development Platform - An Application for Wireless EMG Sensors
73
