
The Computer-aided Diagnostics of Gastric Lesions
by using High Definition Narrow-band Imaging Endoscopy
and Real-time Pattern Recognition System
K. Yu. Erendzhenova
1
, O. A. Kulagina
2
, R. M. Kadushnikov
3
and T. V. Zarubina
1
1
Pirogov Russian National Research Medical University, Ostrovitianov str. 1, 117997, Moscow, Russian Federation
2
Medical Research and Education Center of Lomonosov Moscow State University,
Lomonosovsky prospect 27, korp. 10, 119991, Moscow, Russian Federation
3
LLC “SIAMS”, Kominterna str. 16, 620078, Yekaterinburg, Russian Federation
Keywords: High Definition (HD) Endoscopy, Narrow-Band Imaging (NBI) Endoscopy, Early Gastric Cancer
Diagnostics, Decision Support, Pattern Recognition, Endoscopic Image Processing.
Abstract: High Definition (HD) and Magnified Narrow band imaging endoscopy (ME-NBI) allowed to
recognizetypes of gastric lesions according modified VS-classification by professor Yao K., becausethe
parameters to describe regular or irregularvascular or microsurface pattern and demarcation line in
lesionswere formalized. In this work endoscopic differential criteria of benign and neoplastic epithelial
lesions of stomach were obtained. Based on them classification algorithm for the real-time processing of
narrow–band endoscopic images with a highly productive distributed intellectual analytic decision support
system for multiscale endoscopic diagnostics is presented. We also created the electronic atlas and database
to collect high resolution endoscopic images, applied and proved the differential diagnosis of gastric lesions
through the computer analysis. The algorithm consistentlyused scale– invariant feature transform detector,
computation of gastric mucosa pit–pattern skeletons, “Bag of visual words” method, and K–means method
for key pointsclustering. Resulting classification algorithm is completely automated, performed real-time
analysis, and did not require preliminary selection of interest area. Image classification accuracy was 85%.
1 INTRODUCTION
In Russia stomach cancer takes the first position in the
structure of cancerdiseases (Kaprin, 2017). Every year
tens of thousands of people fall ill with stomach
cancer, and almost half the deaths occur (Savelyev,
2009). Gastric cancer is more often detected in the
late stages of the tumor process. Treatment is
expensive and despite all the efforts of surgeons and
oncologists it often does not prolong the life of such
patients for more than five years. The solution of the
social and economic problem in this direction is the
diagnosis of early gastric cancer (Japanese Gastric
Cancer Association, 1998). Among the instrumental
methods only upper gastrointestinal endoscopy aims
to identify this disorder, as well as benign epithelial
neoplasia and non-neoplastic lesions that are inclined
to atypia.Gastroscopy allows directly visually
evaluate the condition of the gastric mucosasurface.
Correct differential diagnosis of such formations
contributes to the selection of correct treatment tactic
and 95% of patients' survival.
Routine white light endoscopy helps detect
pathology focuses, whereas analysis of a pit and
vascular pattern in mucosal structure, derived from
enhancement techniques such as magnified narrow-
band imaging (ME-NBI) endoscopy with video
endoscopic high-resolution systems, allows to
determine the types of lesions. The variety of these
mucosal changes, the difficulties of their visual
interpretation cause insufficient accuracy of
recognition of pathological processes (Buntseva,
2014). Promising is the creation of an automated
images processing during endoscopic exploration.
Currently endoscopic computer-aided decision–
making systems are created in different countries.
Japanese specialists have achieved the most
advanced results of analysis of thin structure for the
revealed endoscopic lesions. Research by H.
Osawa., H. Yamamoto, Y. Miura, H. Ajibe, H.
Shinhata, M. Yoshizawa, K. Sunada, S. Toma, K.
Satoh, and K. Sugano have shown the efficiency of
computer–aided analysis of endoscopic images using
digital chromoscopy in ascertainment of the
Erendzhenova, K., Kulagina, O., Kadushnikov, R. and Zarubina, T.
The Computer-aided Diagnostics of Gastric Lesions by using High Definition Narrow-band Imaging Endoscopy and Real-time Pattern Recognition System.
DOI: 10.5220/0006724906150620
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 5: HEALTHINF, pages 615-620
ISBN: 978-989-758-281-3
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
615

boundaries between neoplasms and surrounding
mucosa, thereby allowing determination of stomach
cancer flattened forms (Osawa, 2012). R. Miyaki, S.
Yoshida, S. Tanaka, Y. Kominami, Y. Sanomura, T.
Matsuo, S. Oka, B. Raytchev, T. Tamaki, T. Koide,
K. Kaneda, M. Yoshihara, and K. Chayama
examined application of computerized image
processing to endoscopy images obtained using
digital chromoscopy for the purpose of detecting
benign neoplasms and intramucosal early gastric
cancer (Miyaki, 2013). T.C. Lee, Y.H. Lin, N. Uedo,
and H.P Wang presented the previously results of
computer–aided analysis of suspicious gastric
carcinoma images, obtained with narrow band
imaging in (Lee, 2013).Such computer–aided
medical decision-support systems for endoscopy
allow to keep labor costs down for endoscopy
investigation. Nevertheless, theyrequire high level of
endoscopist qualification to adjust analysis settings
for each image. Besides, existing decision–making
systems are not unified; they determine the
particulartype of pathology, do not allow decision-
making during the endoscopic exploration.
Thedisadvantages of the existing decision–support
systems mentioned abovemean that endoscopy
services are very time taking.
With that in mind, the aim of this work was to
develop a decision support system for endoscopic
departments to diagnose precancerous and early
neoplastic changes in the stomach mucosabased on
the intelligent analysis of endoscopic images using
computational methods, which include computer
vision. We propose the method for processing
narrow–band with or without magnification
endoscopic images using a highly productive “Smart
Endoscope” distributed intellectual analytic
decision–support system for multiscale endoscopic
diagnostics and surgery. This system exhibits the
following advantages:ability to learn towards
classify images obtained with various endoscopic
methods; real–time operations that allow to make
decisions on-the-fly, rather than post-test;
completely automated analysis algorithms, which do
not require prior selection of interest areas or
additional operator training.
2 MATERIALS AND METHODS
2.1 Materials
We prospectively selected164 patients with 192
focal superficial epithelial gastric lesions. We
performed 220 HD-NBI and ME-NBI endoscopic
images of lesions surface. In this work we included
prospectively both protruded and flat or depressed
sites of damage. 220 images included 141 photos of
benign lesions (hyperplastic polyps, erosions, ulcers,
focuses of intestinal metaplasia) and 79 photos of
neoplasia (low and high grade intraepithelial
neoplasia, early gastric cancer, invasive and
advanced gastric cancer).So, all images were divided
into two groups according to the tactic of treatment:
First group - non-neoplastic lesions (tactic – is
observation); Second group - epithelial neoplasms of
the stomach (tactic – is minimally invasive
endoscopic,laparoscopic or open surgical treatment)
(Dixon, 2002). The structure of the lesions was
verified by histological examination of biopsy
samples or resected portions of the mucosa.
2.2 Methods
The methods of investigation were routine white
light endoscopy, high definition enhanced narrow
band imaging endoscopy (with magnification from
50 to 115 times).
Statistical analysis included the application of
Fisher's exact test, Cramer's criterion, the
inhomogeneous sequential diagnostic Bayesian
procedure (Gubler, 1978). The significance p-level
was assumed to be 0.05.
On 182 (out of 220) endoscopic images the
method of machine vision "Bag of visual words"
was used to classify benign lesions (n=111) and
epithelial neoplasms of the stomach (n=71)
(Liedlgruber, 2011).
3 RESULTS
3.1 Statistical Analysis
All lesions and images we characterized with 34
criteria. Four criteria were clinical (age, gender,
presence or absence of Helicobacter pylori infection,
primary or residual lesion), 14 – were got during
traditional endoscopy (localization, size, macrotype,
number of lesions,presence or absence of fibrin,
erosionof lesion surface, signs of inflammation,
atrophy or intestinal metaplasia in surrounding
mucosa, consistency, mobility, etc.), 16 – were got
in time enhanced endoscopic explorations. These
sixteen microendoscopic criteria of microsurface and
microvascular pattern included specific features
(such as size, shape) and features by most spreading
classifications (VS-classifications by Yao K. and
Kato, Kaise triad) and combined features.
HEALTHINF 2018 - 11th International Conference on Health Informatics
616
We compared benign and neoplastic lesions by
34 parameters with Fisher's exact test and Cramer's
conjugation coefficient.
Statistical analysis allowed to define 6 significant
endoscopic criteria. On these parameters using the
Bayes procedure, the probabilities of assigning
images to each of the two groups were obtained. The
group classified according to this decision rule was
determined by the maximal probability. For
differentiation benign (n=141) and neoplastic lesions
(n=79) obtained sensitivity was 92%, specificity was
96% and accuracy was 94%. The parameterswere
adopted for clinical use (most significant, easy and
objective endoscopic signs). By comparing groups
of epithelial lesions of the stomach with the exact
Fisher test and the Cramer test, six statistically
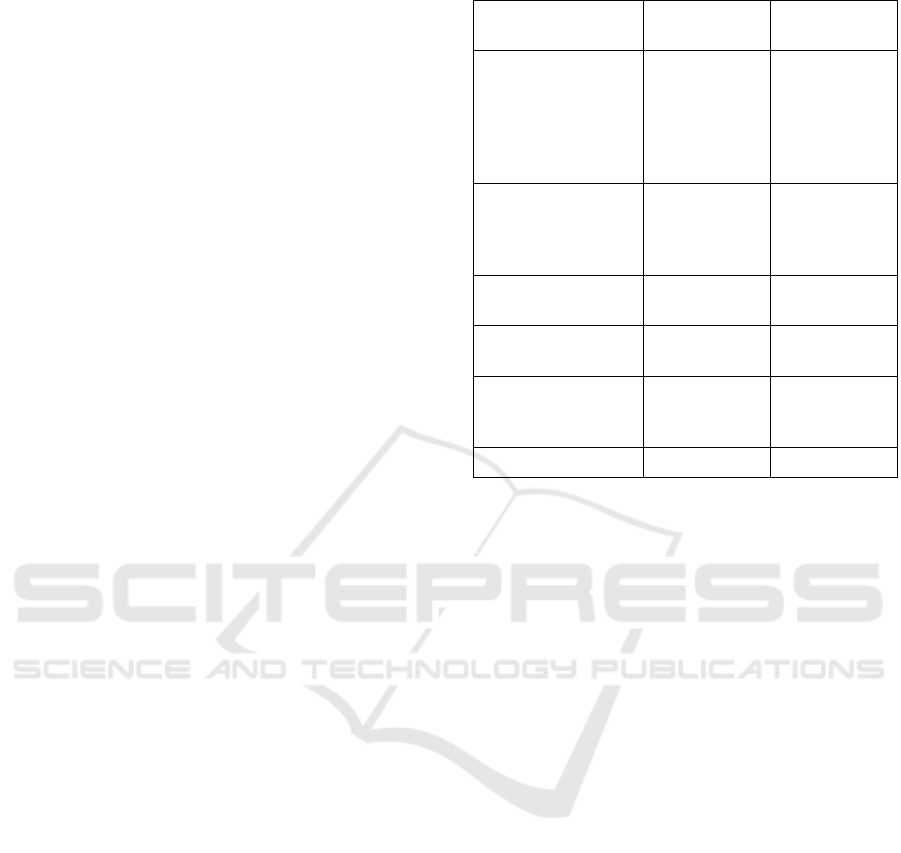
significant parameters were identified (table 1).
The first criterion is the thickness of vascular
component in the lesion compared with
surrounding mucosa. In neoplastic lesions thickness
of vascular component is less or heterogenous than
in surrounding mucosa. And in benign lesions it is
more similar.
The second criterion is the thickness ratio of
glandular and vascular component in the lesion. In
neoplasia thickness of glandular white component is
more than dark vascular component. For benign
lesions thickness of glandular is less or similar to
vascular component.
Next criteria are the thickness and contours of
vascular componentin the lesion. For benign lesions
thickness of vascular component is uniform and
contours are relatively smooth. For neoplasia – they
are highly unequal and uneven.
In neoplastic lesions we can frequently see the
thick findings like blackbright sticks
(individualvessels). In benign lesions, usually, there
are notblack individualvessels.
And the last criterion is the demarcation line.
Similar to VS-classification it is specific for
neoplastic lesions.
The practical check of these criteria in our clinic
showed high accuracy and interobserver agreement.
We checked these 6 microendoscopic features
for differentiation 40 endoscopic images (21 benign,
19 neoplastic lesions) by 3 experts (accuracy was
100%), 2 low experienceddoctors (accuracy was 92-
95%, interobserver agreement (IA) coefficient was
equal 0,75) and 2 inexperienced in HD-NBI
endoscopy doctors (accuracy was 95-98%, IA
coefficient was equal 0,85).
Table 1: Endoscopic differential parameters.
Endoscopic
parameter
Benign lesions
Epithelial
neoplasia
The thickness of
vascular component
(area between the
glands) as compared
with surrounding
mucosa
More or similar
Less or
heterogeneous
The thickness ratio of
glandular (G) and
vascular (V)
component
G is less or
similar than V
G is more than
V
The thickness of
vascular component
Relatively
uniform
Highly unequal
The contours of
vascular component
Relatively
smooth
Highly uneven,
jagged, wavy
The thickened
individual vessels as
bright sticks
No
Yes
The demarcation line
No
Yes
3.2 Real-Time Pattern Recognition
Analysis
The electronic atlas includes white light and
magnified NBI endoscopic images of benign lesions
(hyperplasia, inflammation, atrophy, intestinal
metaplasia), low and high grade intraepithelial
neoplasia, early gastric cancerand advanced stomach
adenocarcinoma. All images with delineated regions
of interest are accompanied by expert’s description
of the clinical parameters, macroscopic and
microscopic structure features of the lesion,
including the vascular and surface patterns, and
histological structure of the lesion.
With the help of the algorithm of computer
vision it became possible to divide endoscopic
images into groups of non-neoplastic lesions of the
mucosa and epithelial gastric neoplasms. The use of
the "Bag of visual words" method for the
mathematical representation of images of focal
superficial epithelial stomach lesions included the
steps of detecting key points (SNoL-detector),
mathematical description (SIFT, Scale-invariant
feature transform descriptor) and clustering of local
characteristics in the key points area (hierarchical k-
means method) and constructing visual words
dictionary (Canny, 1986;Liedlgruber, 2011).
At the first stage, the endoscopic image was
delivered to the automated endoscopist’sworkplace,
where it was transformed in gray levels. Gaussian
blurring was then applied using values of blur radius
The Computer-aided Diagnostics of Gastric Lesions by using High Definition Narrow-band Imaging Endoscopy and Real-time Pattern
Recognition System
617
from the certain range with the present step value.
That produced the “pyramid” of Gaussians.
There are two object types present on endoscopic
images (photos and videos) of stomach mucosa
microstructure – glands, which are the bright areas,
and vessels – dark areas surrounded by glands. Use
of FAGF (Fast Anisotropic Gauss Filtering) to
source images allowedto select of vessels and
glandsbetter (Geusebroek, 2003). At the next
processing step, the image was binarized.
After applying a median filter with a 3x3
window, the pit skeleton wasoutput using the FFPT
(Fast fully parallel thinning) algorithm(Guo, 1992).
The elementary unit of a skeleton is its branch or
rib, which characterizes a pit or a vessel on ainitial
image. Intersections and endpoints of skeleton ribs
become key points of the analyzed image.
Further image processing was performed by
selection and classification of local image features
using the SIFT descriptors(Lowe, 2004). SIFT
descriptors of local image features were built.
Produced local feature descriptors were invariant for
scaling, shifting, rotation and changing illumination
direction. The local feature area relevant to point
blur radius is covered by a 4x4 grid (16 cells in
total). For each grid cell, Histograms of Oriented
Gradients (HOGs) were built for eight directions.
After that, vectors for the cells combined into a
single 128–dimensional vector (8х16), which was
used to describe a key point (Lingua, 2009).
The set of key point vectors was processed using
the “Bag of visual words” algorithm (Liedlgruber,
2011). Histogram of key points distribution by
defined groups was built for each image. Key points
selected by a computer on images were placed into
clustersin a 128–dimensional space using clustering
cloud of points by applying the hierarchical k–
means method. It was found that, for the endoscopic
examinations relevant, the number of clusters should
be equal to 1000. The number of key points that
were placed into each cluster was determined and a
histogram illustrated distribution of the points for
analyzed image by clusters and characterized image
as a wholewas built. The histogram was represented
by a vector in a 1000–dimensional space (vector
coordinate values showed the number of key points
falling into relevant clusters). Image classification
was performed by placing a multidimensional vector
into a classification space produced from the training
set by the Support Vector Machines Method (SVM).
Aimed to test the suggested method, a set of 182
endoscopic images was selected. Images featured
different lesions of gastric mucosa, validated by
means of histological examination. Images were also
obtained using high-resolution narrow–band
endoscopy. There were 111 images of non-
neoplastic gastric epithelial lesions, and 71 images
of different epithelial neoplasms of high and low
grade, of early and advanced gastric cancer. The
scientific and clinical goal for computer-aided
analytic system was to evaluate and differentiate
focal lesions of gastric mucosa as non-neoplastic or
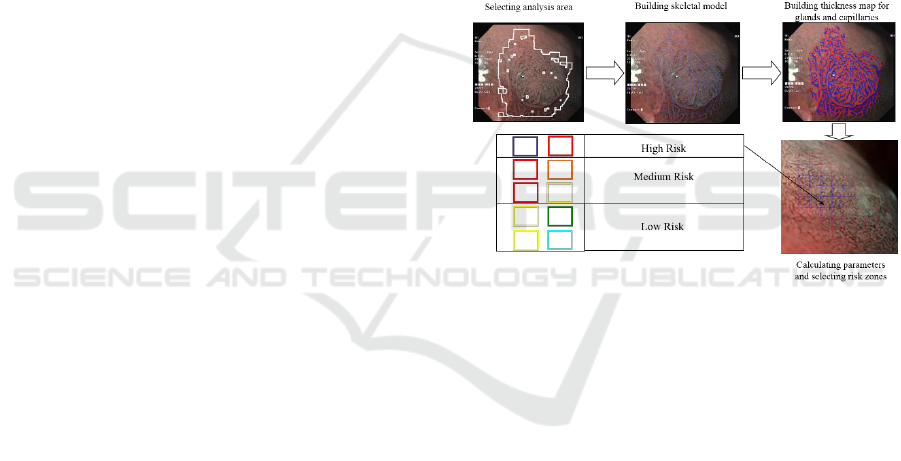
stomach epithelial neoplasms (figure 1).
Recognition algorithm applying results
demonstrate that in 85% of the cases it did perform
correct image classification, placing image into First
orSecond group. Average image analysis time was
less than 30 seconds, and,in fact, that allows using
the algorithm for real-time analysis of endoscopic
images(Stepanov, 2016). A positive assessment of
the current results was approved by practicing
endoscopists.
Figure 1: Classification algorithm for morphological
analysis of capillary and gland microstructure of stomach.
4 CONCLUSIONS
In accordance with the statistical analysis six
microendoscopic features of gastric lesions were
proven most significant for effective differential
diagnosis between benign and neoplastic epithelial
gastric lesions. These features can be successfully
used by nonexperience doctors and also for creating
the decision support system, including through the
computer analysis.
Also, the electronic database will be useful for e-
learning of specialists in gastrointestinal endoscopy
thanks to function of similar endoscopic images
search. However, the main goal of this atlas and
database in future is to provide the direct computer-
aided image analysis during endoscopic
investigation for predicting the histological structure
of the epithelial lesions and choosing the correct
treatment strategy
HEALTHINF 2018 - 11th International Conference on Health Informatics
618

Effective results of application of the decisive
rule and high accuracy of the mathematical
algorithm for the classification of epithelial
neoplasms and non-tumor lesions of the stomach
show the fundamental possibility of formalizing the
microendoscopic structure of the formations, and
hence the possibility of developing their objective
clinical classification and decision support system
for the doctor.
The decision support system with automatic
image identification soon will become an
indispensable part of endoscopic video systems
A method for processing narrow–band
endoscopic images using a highly productive
distributed intellectual analytic decision–making
system was presented. This method allows
improving accuracy and helps avoiding subjectivity
in real-time classification of endoscopic images. It
possesses the following distinctive features:
1. Key points are selected in real time due to
application of FFPT algorithm.
2. Use of SIFT descriptors allows real-time
selection and vectorization of local image
features invariant to scale, shift, rotation and
illumination.
3. Application of the “Bag of visual words”
approach enables processing the whole image
of a mucosal neoplasm (and not just the part
of it examined using histological methods).
4. Use of SVM method for building
classification space.
Examination of a suggested computer algorithm
using 182 endoscopic images todetermine the
neoplasia demonstrated that the accuracy of correct
recognition reaches 85%.
That allows formalization of pit and vessel
pattern descriptors for gastric epithelial neoplasms,
and, in turn, development of a precise and objective
clinical classification. Algorithm efficiency can be
improved by breaking image sets into a larger
number of subgroups according to histological data.
That requires processing wide image set for each
subgroup. It is important that the algorithm is fast
and efficient. That allows using it for processing
video streams and endoscopic images in real time.
ACKNOWLEDGEMENTS
The work was done within the framework of the
project performed by SIAMS Ltd, and supported by
the Ministry of Education and Science of the
Russian Federation (Grant agreement 14.576.21.
0018 dated June 27, 2014. Applied research (project)
UID: RFMEFI57614X0018).
REFERENCES
Buntseva, O. A., Plakhov, R. V., Galkova, Z. V., Fedorov,
E. D., 2014. Modern endoscopic methods of diagnosis
and treatment of precancerous changes and early
gastric cancer.Polyclinic, 2(2), pp. 56-64.
Canny, J., 1986. A computational approach to edge
detection. IEEE Transactions on pattern analysis and
machine intelligence, 6, pp. 679-698.
Dixon, M. F., 2002. Gastrointestinal epithelial neoplasia:
Vienna revisited. Gut, 51(1), pp. 130-131.
Geusebroek, J–M., Smeulders, A. W. M., van de Weijer,
J., 2003. Fast anisotropic Gauss Filtering. IEEE
Transactions on image processing (TIP 2003),
pp. 938–943.
Gubler, E. V., 1978. Computational methods of analysis
and recognition of pathological processes, Medicine.
Leningrad.
Guo, Z., Hall, R. W., 1992. Fast fully parallel thinning
algorithms.CVGIP: Image Understanding, 55(3),
pp. 317–328.
Japanese Gastric Cancer Association, 1998. Japanese
classification of gastric carcinoma - 2nd English
edition. Gastric Cancer, 1, pp.10-24.
Kaprin, A. D., Starinsky, V. V., Petrova, G. V. (ed.), 2017.
Malignant neoplasms in Russia in 2015 (morbidity
and mortality), MSRI named after P.A. Herzen - FGBI
branch of NMRRC of Russian Health Ministry,
Moscow.
Lee, T-C., Lin Y-H., Uedo, N., Wang, H-P., Chang, H-T.,
Hung C-W., 2013. Computer-aided diagnosis in
endoscopy: A novel application toward automatic
detection ofabnormal lesions on magnifying narrow-
band imagingendoscopy in the stomach.In Proc. 35th
IEEE AnnualInt. Conf. of the Engineering in Medicine
and Biology Society (EMBC), pp. 4430–4433.
Liedlgruber, M., Uhl, A., 2011. Computer-aided decision
support systems for endoscopy in the gastrointestinal
tract: a review. IEEE reviews in biomedical
engineering, 4, pp 73-88.
Lingua, A., Marenchino, D., Nex, F., 2009.Performance
Analysis of the SIFT Operator for Automatic Feature
Extractionand Matching in Photogrammetric
Applications. Sensors, 9(5), pp. 3745–3766.
Lowe, D. G. 2004. Distinctive image features from scale-
invariant keypoints. International journal of computer
vision, 60(2), pp. 91-110.
Miyaki, R., Yoshida, S., Tanaka, S., Kominami, Y.,
Sanomura, Y., Matsuo, T., Oka, S., Raytchev, B.,
Tamaki, T., Koide, T., Kaneda, K., Yoshihara, M.,
Chayama, K., 2013. Quantitative identification of
mucosal gastric cancer under magnifying endoscopy
withflexible spectral imaging color enhancement.J.
Gastroenterol. Hepatology, 28(5), pp. 841–847.
Osawa, H., Yamamoto, H., Miura, Y., Ajibe, H., Shinhata,
The Computer-aided Diagnostics of Gastric Lesions by using High Definition Narrow-band Imaging Endoscopy and Real-time Pattern
Recognition System
619

H., Yoshizawa, M., Sunada, K., Satoh, K., Sugano, K.,
2012. Diagnosis of depressed-type early gastric cancer
using small-caliber endoscopy with flexible spectral
imaging color enhancement. Digestive Endoscopy,
24(4), pp 231-236.
Savelyev, V. S., Kirienko, A. I. (ed.), 2009. Clinical
surgery: National guidelines, GEOTAR-Media.
Moscow.
Stepanov, D. M., Mizgulin,V. V., Kosulnikov, V. V.,
Kadushnikov, R. M., Fedorov, E. D., Buntseva, O. A.,
2016. Detector of interest point within region of
interest on NBI endoscopyimages.In Proc. Analysis of
Images, Social Networks, and Texts.
HEALTHINF 2018 - 11th International Conference on Health Informatics
620
