
Building IoT-Enabled Wearable Medical Devices: An Application to a
Wearable, Multiparametric, Cardiorespiratory Sensor
Arthur Gatouillat
1
, Bertrand Massot
2
, Youakim Badr
1
, Ervin Sejdi
´
c
3
and Claudine Gehin
2
1
Univ. Lyon, INSA-Lyon, LIRIS, UMR5205, F-69621, France
2
Univ. Lyon, INSA Lyon, INL, UMR5270, F-69621, France
3
Department of Electrical and Computer Engineering, Swanson School of Engineering,
University of Pittsburgh, Pittsburgh, PA, U.S.A.
Keywords:
Wearable Sensor, Medical IoT, Heart Rate Sensor.
Abstract:
Recent developments in personal and mobile healthcare have shown promising results in term of patients’ quality
of life and quality of care improvements. This can be achieved through continuous monitoring of patients’
physiological functions using wearable non-invasive biomedical sensors. The remote collection and processing
of such data can then be used to provide rapid medical response if a problem is detected or to offer preventive
measures. However, the integration of wearable sensors into wider-scale framework is still a major challenge,
as real-time data collection and remote configuration capabilities must be integrated to strongly constrained
devices. Here, we show how such requirements can be integrated into a multiparameter, cardiorespiratory
wearable sensor and how this sensor can be integrated into wide-scale Internet-based frameworks. We thus
manufactured a biomedical-grade heart rate, instantaneous heart-rate variability and respiratory sensor. The
sensor was tested in real life ambulatory condition, and we showed an Internet-based proof of concept exhibiting
the integration of our sensor into wide-scale healthcare frameworks. Finally, we anticipate that wearable
healthcare will greatly improve patients’ quality-of-life by using IoT-based wearable devices similar to the
sensor developed in this paper.
1 INTRODUCTION
Personalized and mobile healthcare are growing fields
of interest in the biomedical community. The idea
behind such concepts is to provide patients with health
recommendations and diagnostic tailored to their indi-
vidual needs. This can be achieved using continuous
remote monitoring of the patients’ physiological func-
tions associated with data analysis to offer preventive
measures, or rapid medical response if a physiologi-
cal malfunction is detected. Consequently, personal-
ized healthcare is a promising approach to improve
the financial and therapeutic efficiency of healthcare
(Van Hoof and Penders, 2013), by avoiding unneces-
sary hospitalization while preserving the safety of the
patients.
Personalized healthcare can be achieved using
wearable sensors networks (Massot et al., 2013), which
are used to accurately monitor vital signs. Cardiac
health estimation devices are of particular interest be-
cause of the crucial nature of the cardiac function and
because it has been well explored by the biomedi-
cal sensor community, for instance in (Massot et al.,
2015), (Magno et al., 2014), (Altini et al., 2011),
(Khayatzadeh et al., 2013) and (Tuominen et al., 2017).
Moreover, cardiac activity parameters can be used as
an indicator of several pathologies. In particular, heart
rate variability (HRV) provides insights about the au-
tonomic nervous system parameters which reflects the
patients’ emotional state (Task Force of the European
Society of Cardiology the North American Society
of Pacing Electrophysiology, 1996). However, one
of the major research problem at the moment is the
integration of developed wearable sensors into bigger
frameworks. Indeed, personalized healthcare assumes
health data to be collected in real-time and that such
data is analyzed by powerful algorithms on distant
servers. Personalized healthcare also makes remote
sensor reconfiguration necessary in order to better suit
both patients and healthcare professional expectations.
All the identified wearable electrocardiogram-
based cardiac activity sensors, despite accurately mea-
suring cardiac activity parameters, do no feature
enough connectivity and functionality to be able to
Gatouillat, A., Massot, B., Badr, Y., Sejdi
´
c, E. and Gehin, C.
Building IoT-Enabled Wearable Medical Devices: An Application to a Wearable, Multiparametric, Cardiorespiratory Sensor.
DOI: 10.5220/0006729101090118
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 1: BIODEVICES, pages 109-118
ISBN: 978-989-758-277-6
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
109

use them in a personalized healthcare context. Only
wearable ECG or ECG-based cardiac parameters sen-
sors were studied because of their accuracy in terms
of cardiac parameters estimation. Although widely
used, the accuracy of other popular methods such as
plethysmography is still questioned by the community
(Sch
¨
afer and Vagedes, 2013) and suffer from heavy
movement artifact. Authors of (Magno et al., 2014) de-
signed a wearable heart rate and respiratory rate sensor
with Bluetooth low energy and proprietary 802.15.4
connectivity by combining several development kits.
This solution lacks remote configuration capabilities
and proper integration, which both are critical aspects
of personalized healthcare. In (Tuominen et al., 2017),
a remotely configurable ECG sensor was designed,
but data is stored locally on a SD card, which makes
this solution unsuitable for remote healthcare applica-
tions. Another wearable ECG sensor was developed
by (Izumi et al., 2015), is remotely configurable, and
data are sent using wireless connectivity. However,
this device use near field communication (NFC) for all
of its connectivity. The short range of NFC application
makes it unpractical for real life data collection, as data
can only be collected when a NFC reader is brought in
proximity of the sensor. A heart rate (HR) and HRV
sensor is described in (Massot et al., 2015). This de-
vice offers on-board cardiac parameters calculation,
and computed parameters are sent to a smartphone
using wireless connectivity. However, this sensor is
not remotely configurable, and data is only stored lo-
cally on the smartphone, which makes some adaptation
necessary in order for this device to be used in a per-
sonalized healthcare concept.
Recently, the study of the interconnection of mul-
tiple devices featuring real-time data collection and
remote configuration capabilities was studied under
the scope of the Internet-of-Things (IoT) (Gubbi et al.,
2013). In the IoT, a variety of heterogeneous com-
municating devices are interconnected, but also com-
municate with external services implemented on re-
mote servers. Consequently, the IoT seems like an
approach of particular interest when trying to solve the
challenges of wide-scale personalized healthcare (Fer-
nandez and Pallis, 2014), and this paper will describe
the integration of IoT characteristics on a biomedical
wearable cardiac and respiratory sensor to enable its
integration into a wide scale personalized healthcare
framework.
Our solution focuses on the integration of remote
reconfiguration capabilities to our sensor, the imple-
mentation of different functional modes to enable
adaptive capabilities, and the real-time streaming of
biomedical data. All these functionalities must pre-
serve measurements accuracy. The impact of integrat-
ing IoT characteristics to the sensor on both hardware
and software designs, but also on the overall consump-
tion of the device was carefully studied in order to
ensure our sensor validity from a real-life perspective.
The following paper is organized as follows. Sec-
tion 2 introduces the materials and methods enabling
the integration on IoT properties to a biomedical grade
sensor, but also describes the design of analog/digital
electronics to achieve the desired functional and non-
functional goals. In section 3, we present a compre-
hensive caracterization of the sensor from a power
consumption and data quality evaluation perspective,
and finally section 4 discusses the potential application
of such sensor for patients and medical practitioners.
2 MATERIALS AND METHODS
The manufactured IoT-enabled wearable cardiac and
respiratory activity sensor is displayed in Figure 1.a.
This circuit board is small (
40 mm × 20 mm × 6 mm
),
and it is packaged along with a
300 mAh
battery in a
plastic enclosure. The complete sensor (i.e., PCB, bat-
tery, plastic enclosure and cables) weighs only 26.7 g,
making it light enough for wearable applications.The
sensor is designed to be used with three electrodes:
left arm, right arm and a common mode rejection elec-
trode, as illustrated in Figure 1.b. The left arm and
right arm electrodes are connected to the sensor us-
ing wires, and the sensor is directly attached to the
common mode rejection electrode. Consequently, the
device can be comfortably worn by the patients for
extended periods of time. The battery of the sensor is
charged using the micro-USB port, and the sensor can
be reset to its default state using the single push-button
of the device. This sensor is able to measure both
the heart rate, heart rate variability parameters and the
respiration waveform (RWF).
2.1 Sensor Hardware Description
The global sensor architecture is given in Figure 2.
Because of the potential overhead due to the addition
of IoT-related characteristic to our device, components
were selected in order to maximize their computational
and power efficiency.
In order to minimize CPU load and CPU wake-up
time caused by real-time signal processing, it must
be performed using dedicated hardware. This led to
the choice of a PSoC 5LP (Cypress Semiconductor,
San Jose, CA) for the device microcontroller and ded-
icated signal processor. Indeed, this integrated cir-
cuit (IC) offers both an ARM Cortex Core M3 CPU
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
110
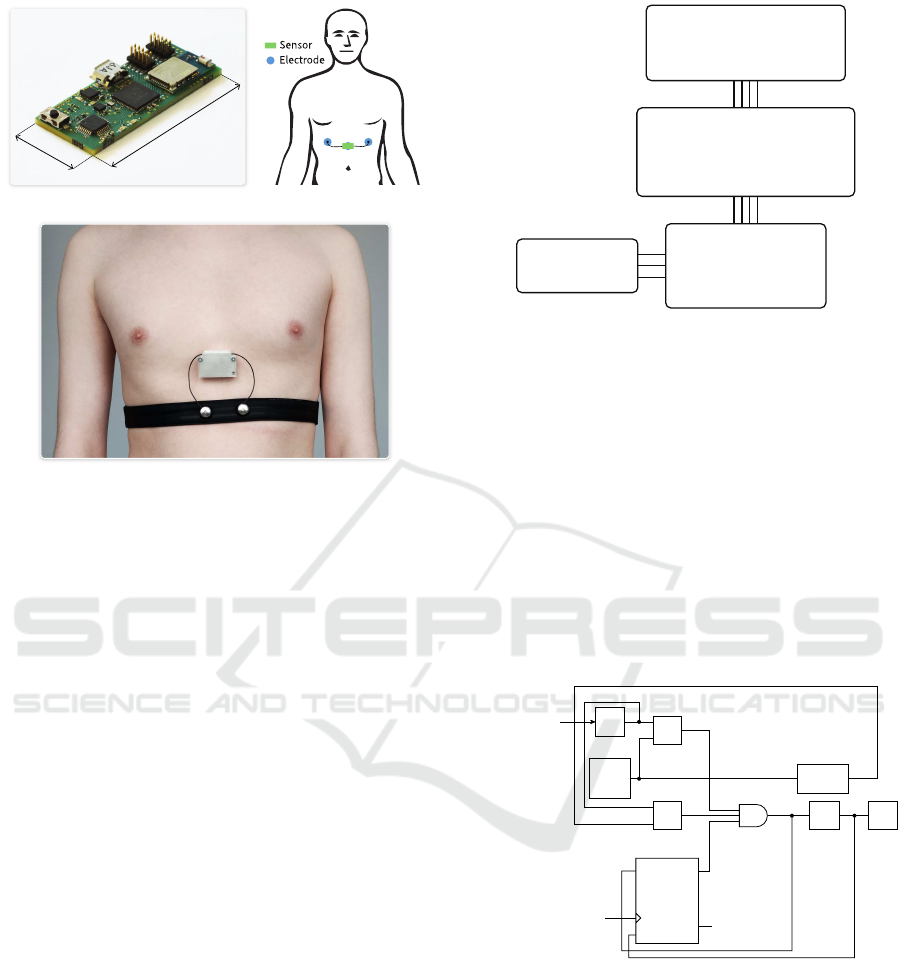
40 mm
20 mm
(a) (b)
(c)
Figure 1: Manufactured sensor (a), body placement (b) and
sensor worn in ambulatory conditions (c).
core and a programmable digital filter block in a sin-
gle package, leading to smaller PCB, thus improving
the comfort of the patients. Because Bluetooth Low
Energy (BLE) is the main protocol used in resource
constrained devices for the IoT and because of its
widespread adoption (Harris III et al., 2016), it was
selected to provide wireless connectivity to our sen-
sor. The BLE113 integrated chip (Silicon Labs, Austin,
TX) was selected as the BLE dedicated microprocessor
because it integrates both a full hardware and software
BLE stack with integrated antenna design in a small
15.75 mm × 9.15 mm × 1.9 mm
package. This inte-
grated circuit is fully programmable, and all commu-
nication dedicated firmware can be deployed on this
IC, thus reducing the microprocessor computing load.
In order to be able to simultaneously measure both the
ECG and the respiration waveform, the ADS1292R
(Texas Instruments, Dallas, TX) was selected. This
low-power analog front-end (AFE) integrates two dif-
ferential amplifiers and two 24 bits analog to digital
converters. It also features a right-leg drive (RLD) am-
plifier to implement common mode rejection (Winter
and Webster, 1983) along with lead-off and respiration
signal modulation and demodulation circuitry. The
measurements are sent using a SPI bus at a config-
urable sampling rate (from 125 to 8000 samples per
seconds).
In the default state, the analog front end is con-
figured to measure both the respiration waveform on
channel 1 and the ECG signal on channel 2 at a sam-
Analog front-end
Signal amplification
Respiration measurements
Signal filtering
Analog-to-digital conversion
Bluetooth module
Advertising
Connection/Disconnection handling
Data communication
ADS1292R
BLE113
Disposable ECG
electrodes
Digital bus
Programmable system-on-chip
Hardware ECG processing
Soware parameters computation
IoT state machine
Battery level monitoring
PSoC5LP
Digital bus
Figure 2: Hardware architecture of the sensor.
pling rate of a 1000 SPS using the internal clock of the
IC. This sampling rate enables a
1 ms
resolution for RR
interval measurements. The RLD amplifier and lead-
off detection circuitry are both enabled, along with the
respiration modulation and demodulation modules.
The RR interval computation is implemented us-
ing hardware filtering and peak detection. The digital
filter block of the PSoC5LP is used to compute the
smoothed moving average derivative of the ECG sig-
nal, which is then used in combination with adaptive
thresholding in order to compute the RR interval. De-
tails about the implementation are given in Figure 3
and in (Massot et al., 2016).
>
<
count
capture
reset
comp
value
Up Counter
Sampling CLK
1 kHz
DMA1
Trig.
Int.
Counter value
tranfered to RAM
with DMA1
DFB
Delayed
DFB
Output
Adaptative
Threshold
ECG signal from AFE
transfered with
external DMA
Figure 3: Hardware detection of RR intervals.
Using this hardware-centric approach to perform
computationally intensive real-time signal processing
reduces the CPU load and increases the CPU sleep
time, thus reducing global power consumption. All the
other sensor functional tasks are implemented using a
software approach, and will thus be further described
in the following section.
Building IoT-Enabled Wearable Medical Devices: An Application to a Wearable, Multiparametric, Cardiorespiratory Sensor
111

2.2 Sensor Software Description
The firmware of the sensor was developed to achieve
several functional and non-functional goals. The first
goal is focused on the measurements and their trans-
mission: the real-time communication of the instanta-
neous heart rate, the computation and communication
of HRV parameters every 5 minutes, the real-time com-
munication of 6 respiration samples every second. The
second goal is to transmit non-functional information
about the sensor, typically the battery level or if the
sensor is unattached. Finally, the last goal is the im-
plementation of IoT dedicated software, which is used
to integrate the sensor into a wider IoT-based frame-
work. Such software is used to transmit information
about the sensor state in order to trigger remote sensor
configuration.
2.2.1 Measurements Dedicated Software
This firmware is implemented on the PSoC5LP, and
it can be divided into three sections: the computation
of the instantaneous heart rate when a heart beat is
detected (i.e., when an interruption is triggered by the
peak detection hardware). The heart rate can be easily
computed using the following equation:
HR
BPM
=
60 × SR
RR
int.
=
60 × 1000
RR
int.
(1)
where
HR
BPM
is the instantaneous heart rate in beats
per minutes (BPM),
SR
is the sampling rate in samples
per seconds and
RR
int.
is the RR interval in samples.
This computation is implemented directly on the mi-
crocontroller and the RR interval values are buffered
to be used in the HRV parameters computation.
Every 5 minutes, the HRV parameters are com-
puted from the buffered RR interval values in accor-
dance with the recommendations of the joint Task
Force of the European Society of Cardiology (Task
Force of the European Society of Cardiology the North
American Society of Pacing Electrophysiology, 1996),
and these parameters are summarized in Table 1.
The computation of the frequency domain pa-
rameters are based on the power spectral density
(PSD) estimation. Because the RR intervals signal
is unevenly sampled, the Lomb-Scargle periodogram
(Lomb, 1976) was determined using the fast computa-
tion algorithm developed by (Press and Rybicki, 1989).
We used the same optimal algorithmic parameters than
(Massot et al., 2016). Indeed, authors of (Massot et al.,
2016) conducted a systematic evaluation of the impact
of the parameters on the trade-off between computa-
tion time and periodogram precision, resulting in the
proposition of optimal parameters with respect to both
the periodogram precision and the computation time.
Table 1: Computed HRV parameters
Variable Unit Domain Description
SSDN ms Time Standard deviation of buffered RR
intervals
RMSSD ms Time Quadratic mean of differences be-
tween consecutive RR intervals
LF/HF n.u. Freq. Low-frequency (0.04 to 0.15 Hz)
to high-frequency (0.15 to 0.4 Hz)
components ratio of the PSD of the
buffered intervals
Norm. LF % Freq. Normalized low-frequency compo-
nents to sum of low- and high-
frequency components of the PSD
ration, i.e., LF/(LF+HF)
The respiration waveform is buffered in the RAM
of the PSoC5LP, and is transmitted wirelessly every
second. The first step of the respiration signal pro-
cessing is the downsampling of the respiration sig-
nal from 1000 SPS to 6 SPS. A sampling frequency
of 6 Hz, resulting in a Nyquist rate of 3 Hz, is well
within the bandwidth of respiratory signals, which is
at most 1.5 Hz (Zhao et al., 1994), and decreasing the
sampling rate reduces energy consumption because of
the smaller amount of transmitted information. Af-
ter signal downsampling, the respiration waveform is
smoothed using an exponential filter:
y[n] = (1 − α)y[n − 1] − αx[n] (2)
with
α
a configurable filter parameter. For the prelim-
inary results, this parameter was set to
0.5
. Once the
signal is filtered, the value are communicated to the
Bluetooth IC using a signed 24 bits integer format.
2.2.2 Communication Dedicated Software
In this section, the firmware of the BLE113 IC will be
detailed. The scripting language provided by Silicon
Labs, BGScript, was used to program this firmware. A
custom GATT server was implemented with respect
to the heart rate profile detailed in the Bluetooth 4
specification: the mandatory generic access profile, de-
vice information, heart rate and battery services were
implemented. The heart-rate service was augmented
with a customized heart rate variability characteris-
tic. This characteristic is of length 5, and the SSDN,
RMSSD, LF/HF ratio and normalized LF components
ratio are encoded as single-byte unsigned integers. An
additional service was implemented for the respiration
along with another service dedicated to IoT-related
information (i.e., information about the sensor non-
functional states).
The BLE113 also implements device advertise-
ment when the sensor is not connected and if a discon-
nection occurs according to the heart rate profile: the
advertising interval is between 20 and 30 ms for the
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
112

first 30 seconds, and it is increased to be between 1
and 2.5 s for the next 30 seconds. If no device connect
to the sensor after this advertising period, the sensor is
put in a very low-energy deep sleep mode.
2.2.3 IoT Dedicated Software
To enable our respiratory and cardiac activity sensor
with IoT-based characteristics in order to include the
object in wider IoT-based frameworks, the require-
ments are three-fold:
•
The data must be streamed in real time, meaning as
soon as a data is acquired, it must be transmitted.
•
The sensor must be able to provide information
about its functional and non-functional state in
order to be able to build smart-scenarios based on
the IoT-enabled devices.
•
The sensor must be remotely configurable in or-
der to be integrated to wider-scale auto-adaptation
scenarios.
In order to fulfill this set of requirements, the sen-
sor was modeled using a labeled transition system
(LTS). LTSs are widely used models of computation
(MoC) because of their relative simplicity and high
expressiveness.
The LTS of our sensor is given in Figure
??
. The
transitions between the states are either controllable
(i.e., a remote tier can force the transition) or non-
controllable (i.e., the sensor automatically determines
its state from environmental measurements). As a
convention, a transition written as “
a \ b
” means the
transition is triggered on detection of internal event
a
or is remotely triggered using event
b
. When a transi-
tion is only labeled with a single event, it is assumed
that this event is internal.
As displayed in Figure
??
, the sensor has 5 non-
functional states:
•
When turned on or after a reset operation, the sen-
sor is in the
initialization
state. In this state, the
AFE is configured with the default parameters and
the BLE113 is software reset. This state automat-
ically calls the normal operation state when the
initialization process is over.
•
The default state is the
normal
state. In this state,
both ECG and respiration signal are acquired. The
instantaneous heart-rate is sent in real time (i.e.,
the new value is transmitted as soon as it is avail-
able), the HRV parameters are computed every 5
minutes, and the respiration signal is packaged and
transmitted every second.
•
The
failsoft
state is an energy saving state. It is
triggered either externally or when the battery level
of the sensor reaches a low level (i.e., 20% in our
case).
•
When the sensor detects a disconnected lead, it
is placed in the
unattached
state. In this state, a
timer is launched, and the sensor is turned off if
the timer overflows. If the sensor is reattached, it
goes back to the normal operation state.
•
The
stop
state denotes a very low-energy deep
sleep mode. The sensor can be placed in this state
at any time.
The BLE113 can generate “no connection” (abbre-
viated as “no conn” in the LTS) events if the sensor
does not receive any connection request during the
advertising interval. This causes the sensor to go into
the very low power stop state.
Init.
Normal FailsoUnatt.
Stop
init_done
batt < 20% \ setMode(failso)
batt ≥ 20% \ setMode(normal)
(batt < 5% ∨ no_conn) \ setMode(stop)
no_conn \ setMode(stop)
(timeout ∨ no_conn) \ setMode(stop)
hardware_reset
unattached
unattached
Figure 4.
Practically, this LTS is implemented on the ARM
core of the PSoC5LP. Events are either generated in-
ternally or transmitted by the BLE113, and the sensor
state is exposed using a custom service.
2.3 Gateway Software and IoT-Based
Framework
Android phones were chosen as the main gateways
to our sensor. This choice was motivated principally
because phones can be carried by the subjects, result-
ing in a mobile gateway with perpetual connection to
the Internet. Consequently, an Android application
was developed to connect to the sensor, but also to
plot the data, store it locally on the phone or trans-
mit them over the Internet. The application is based
on the nRF Toolbox open-source application
1
devel-
oped by Nordic Semiconductor (Oslo, Norway), and
screenshots of the application are given in Figure 5.
Because data is collected in real time and sensor
configuration can be triggered at any moment, a pub-
lish/subscribe type protocol was chosen. Practically,
1
https://github.com/NordicSemiconductor/Android-
nRF-Toolbox
Building IoT-Enabled Wearable Medical Devices: An Application to a Wearable, Multiparametric, Cardiorespiratory Sensor
113

Figure 5: Companion Android application.
the Eclipse Paho MQTT client was integrated in the
application. MQTT is a lightweight publish/subscribe
messaging Internet-based protocol. It was designed to
be used in memory and bandwidth constrained devices.
This protocol is built around the notion of topics. A
topic is an URI-like string used to describe transiting
data. In the MQTT terminology, a publisher sends data
in a topic. If a subscriber subscribes to the same topic,
it will receive the data instantaneously. Formally, all
data is sent through a central broker that can be seen as
a hub distributing data between the relevant publishers
and subscribers. This publish/subscribe mechanism
thus implements real-time data streaming over the In-
ternet, and enables connection to various external Web
services or other connected devices.
3 RESULTS
3.1 Power Consumption
Characterization
In this section, a comprehensive evaluation of the sen-
sor power consumption with respect to connection
parameters and non-functional IoT-state defined in Fig-
ure 4 is performed.
Current consumption measurements were per-
formed using a Keithley 2400 sourcemeter (Beaverton,
OR, USA). The first characterization to be performed
was a static power consumption evaluation of the nor-
mal, failsoft, and stop states. The initial state was not
measured because it is only a transitional state with a
short duration, and the unattached state was not mea-
sured because the sensor is in the same hardware con-
figuration than in the normal state, resulting in equal
power consumption. This static characterization was
performed using default Android 7.0 BLE connection
parameters (i.e., connection interval of
48.75 ms
, time-
out of
20 s
and latency of 0). The consumption results
are given in Table 2. With a power consumption of
10.53 mW in the normal state, the battery life of the
sensor if of about 75 hours using a 300 mAh battery.
This battery life is extended to about 85 hours if the
failsoft mode is used. In the stop mode, the sensor can
last more than two months on a fully charged battery.
Table 2: Static power characterization of IoT states.
IoT state Power consumption (mW)
Normal 10.53
Failsoft 9.18
Stop 0.505
In order to further optimize power consumption
of the sensor, the influence of the BLE connection
parameters were also studied. There are three BLE
connection parameters: interval, latency and timeout.
The connection interval designates the period between
two master’s requests to the slave, while the latency
defines the number of connection intervals that can
be ignored safely by the slave. Finally, the connec-
tion timeout designates the period after which the BLE
master will consider that the connection with the slave
is lost. It is only after the timeout period that the BLE
master can attempt with reconnection. For the sensor,
we fixed both the connection interval and connection
timeout, and the impact of the latency was studied on
the overall power consumption. The results of this
characterization are given in Figure 6, and the con-
sumption of both the normal IoT state and the failsoft
IoT state are plotted against the latency for various
connection interval and connection timeout. For all
states and connection interval and timeout parame-
ters, and increase in the latency results in decreasing
overall power consumption, with a stronger decrease
for smaller latency values. It is worth noting that for
a latency of 8, connection instability was observed.
In order to enable smaller power consumption, while
keeping acceptable connectivity quality, the sensor
requests a latency of 2 when a new connection is es-
tablished. However, masters (such as Android phones)
can reject connection parameters update and force a
new set of less constrained connection parameters (i.e.,
smaller connection intervals, zero latency and smaller
connection timeout), causing an increase in the overall
power consumption. This problem was solved with a
slight oversizing the battery capacity in order to always
achieve at least 48 hours of battery life.
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
114
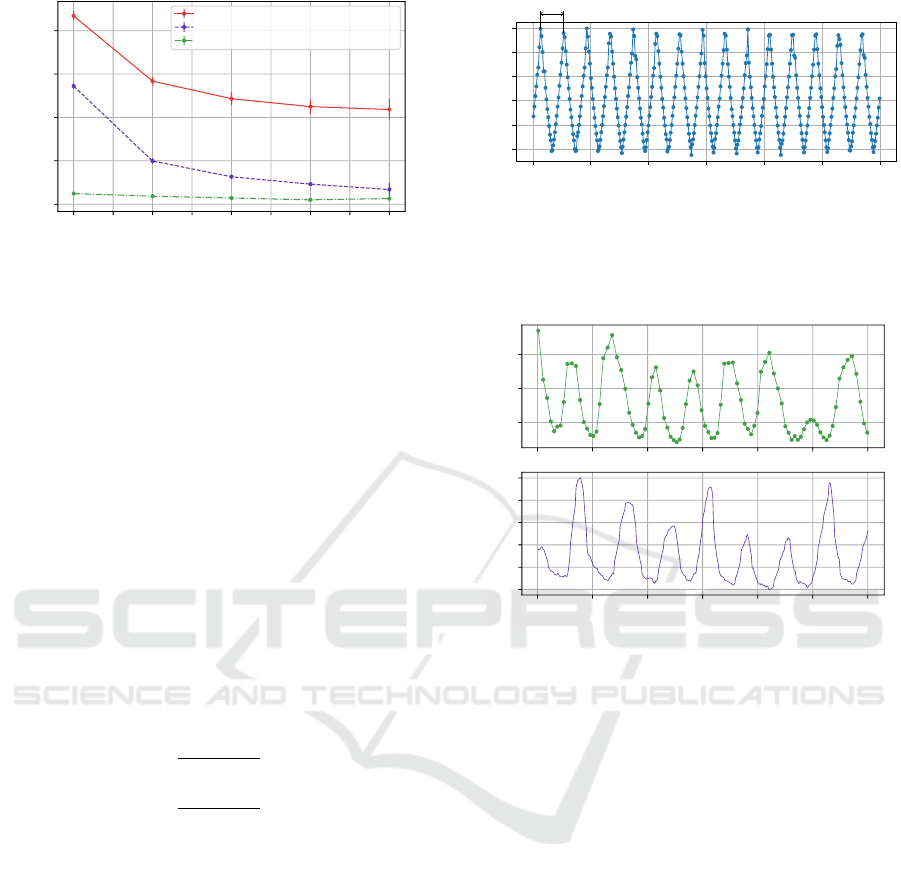
0 1 2 3 4 5 6 7 8
Latency
6.5
7.0
7.5
8.0
8.5
Sensor power consumption (mW)
Normal mode, interval = 67.5 ms, timeout = 1.0 s
Failsoft mode, interval = 67.5 ms, timeout = 1.0 s
Failsoft mode, interval = 742.5 ms, timeout = 10.0 s
Figure 6: Characterization of the BLE connection latency
parameter effect on the overall power consumption.
3.2 Data Acquisition
In this section, various data acquisition are presented.
This first experiment to be performed was the valida-
tion of RR-interval acquisition using synthetic ECG
signals generated using an Agilent 33220A (Santa
Clara, CA, USA) arbitrary waveform generator. The
objective of this test is twofold: it validates the com-
putation of RR-interval and the reliability of the BLE
connection. In order to simulate heart rate variability,
triangular frequency modulation was applied to the
synthetic ECG signal:
base frequency = 1.25 Hz
frequency deviation = 200 mHz
(3)
which theoretically results in RR intervals ranging
between:
RR
int
<
1000
1.25 − 0.2
≈ 952.4 ms
and, RR
int
>
1000
1.25 + 0.2
≈ 689.7 ms
(4)
The sensor was then connected to a Raspberry Pi
used as a BLE data logger to record RR-intervals and
HRV parameters, and an excerpt of the collected RR
interval values is given in Figure 7. The experiment
was performed during 1 day, 17 hours and 9 minutes.
A total of 185153 were logged. Data was analyzed
for records violating the theoretical limits (with a tol-
erance of 1% over the limits), and no violations were
found.
The next experiment was data acquisition during
short respiratory exercises. During this exercise, a
young healthy patient was asked to perform a series
of forced inspiration and expiration during 1 minute
in order to trigger sinus arrhythmia. Results from
this experiment are given in Figure 8, where both the
instantaneous heart rate and the normalized respiratory
waveform are plotted. This figure clearly illustrates
sinus arrhythmia, with increasing heart rate during
228900 228950 229000 229050 229100 229150 229200
time (s)
700
750
800
850
900
950
RR Interval (ms)
20.0 s ⇔ 50 mHz
Figure 7: RR interval determined from synthetic ECG signal.
inspiration because of a decreasing vagal tone and
decreasing during expiration.
600
700
800
RR Interval (ms)
60 70 80 90 100 110 120
time (s)
0.0
0.2
0.4
0.6
0.8
1.0
Normalized RWF
Figure 8: RR-Interval and respiratory waveform during con-
trolled respiratory exercise.
The final experiment consisted on a short-term am-
bulatory testing of the sensor. For this experiment, a
patient was asked to wear the sensor during one hour,
while performing normal daily activities. Cardiorespi-
ratory data was recorded using the companion Android
application, and results from this experiment are given
in Figures 9 and 10. Figure 9 displays both the instan-
taneous RR interval and normalized respiration signals.
No measurement artifact were observed for both the
signals. ECG artifacts, resulting in RR interval arti-
facts, are typically caused by electrical muscular activ-
ity interfering with ECG signals. Such adverse effects
were minimized by placing on the lower part of the rib
cage. Indeed, this location features minimal muscle
thickness, thus reducing interactions between ECG sig-
nal and electrical muscular activity. It is worth noting
that the respiration signal features a slowly evolving
component. This is because the respiratory activity is
in fact an impedance measurement, and because the
impedance of the electrode-skin interface can slowly
evolve over time because of various factors (such as
humidity, the presence of sweat, etc.). This can be
corrected by applying a high-pass filter during signal
post-processing.
Building IoT-Enabled Wearable Medical Devices: An Application to a Wearable, Multiparametric, Cardiorespiratory Sensor
115
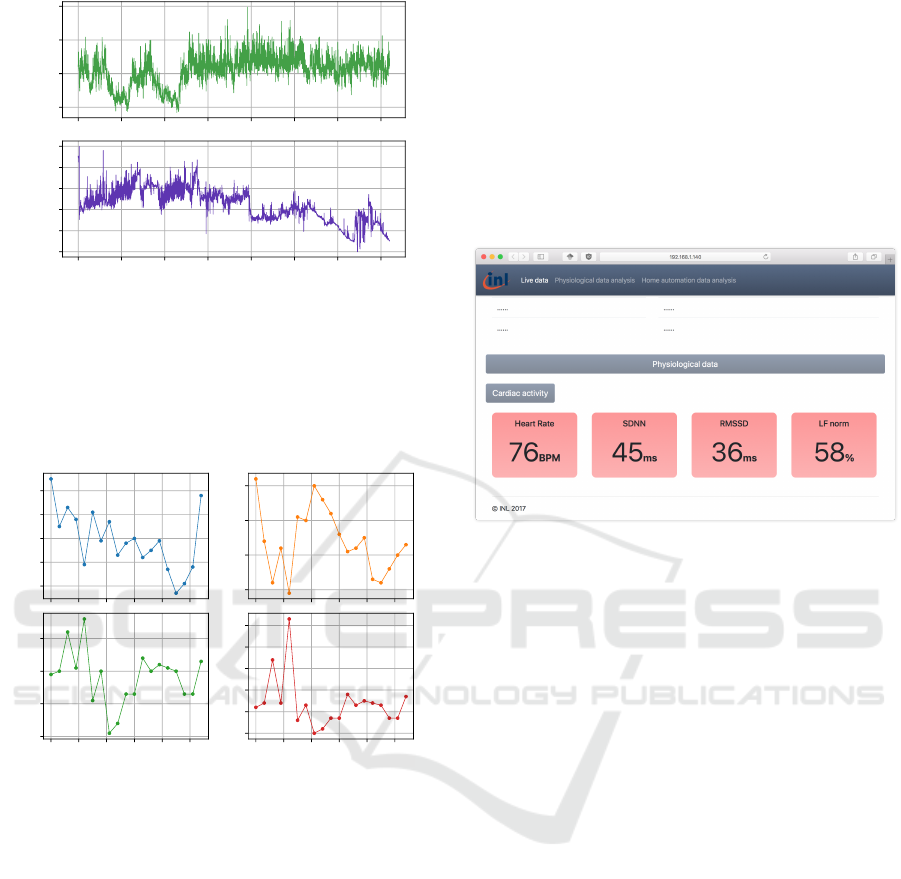
400
600
800
1000
RR Interval (ms)
0 500 1000 1500 2000 2500 3000 3500
time (s)
0.0
0.2
0.4
0.6
0.8
1.0
Normalized RWF
Figure 9: Real-life RR-Interval and respiratory waveform.
Figure 10 displays the 4 heart rate variability pa-
rameters plotted as a function of time. These HRV
parameters correspond to the HR measurements ex-
hibited in Figure 9, and fall within the typical ranges
defined by the literature (Nunan et al., 2010).
40
50
60
70
80
SDNN (ms)
20
30
40
50
RMSSD (ms)
0 1000 2000 3000 4000 5000
time (s)
50
60
70
80
LF n.u. (%)
0 1000 2000 3000 4000 5000
time (s)
1
2
3
4
5
6
LF/HF
Figure 10: Real-life HRV parameters.
The preliminary results presented herein-above
proves that our wearable sensor provides high quality
data. The next experiment will deal with the integra-
tion of our wearable sensor in wider-scale IoT-based
healthcare framework.
3.3 Integration to an IoT-Based
Framework
As specified in Section 2, the integration of our wear-
able sensor to wider-scale healthcare frameworks oc-
curs through the Android companion application. In-
deed, upon sensor connection, the application attempts
to connect to a local MQTT broker. If the connection
is successful, the application will publish heart rate
and HRV values as soon as they are available on two
topics:
• interface/hr
• interface/hrv
As a result, cardiac parameters are streamed in real
time using these two topics, and this is the basis to
build wider-scale healthcare framework. As a proof
of concept, a monitoring Web-based graphical user
interface was implemented. It simulates the kind of
GUI that can be made available to physicians in order
to have real time health information on their patients.
A sample screenshot of the Web-based GUI is given
in Figure 11.
Figure 11: Web application visual.
4 DISCUSSION
In this paper, we introduced a wearable cardiorespira-
tory sensor that can be easily integrated to wider-scale
healthcare framework. This sensor satisfies crucial
healthcare-related requirements such as data realia-
bility, remote configuration capabilities or scalability.
Wearable healthcare and the Internet-of-Things have
similar objectives: the use of a mass of connected ob-
jects (which also are worn by patients in the context
of wearable healthcare) that monitor physical parame-
ters in order to trigger medical advice or intervention
based on data analysis. For instance, our wearable sen-
sor can be integrated in a living-lab, where the living
environment of the patient is continually monitored.
This integration relies on the use of widely adopted
technologies such as BLE, but also on Internet-based
technologies such as MQTT in order to enable wide-
scale connectivity. Internet-based connectivity implies
that our wearable sensor is able to connected to Inter-
net healthcare services, which can perform advanced
data analysis and detect potential health crisis of the
patient and trigger urgent medical response.
Wearable healthcare have a strong potential on the
improvement of the patients’ quality of life and quality
of care. Indeed, the wearable nature of devices en-
able patients to be continuously remotely monitored.
Combined with recent advances in home-automation,
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
116

wearable healthcare will drastically improve in-home
care for a variety of patients. Collected data processed
using big-data techniques or advanced signal process-
ing could also have great predictive values for the
evolution of chronic diseases and could be used to
provide better and earlier care for patients.
However, the personal nature of collected health
data mandates strong security mechanism, which were
not explored in our contribution. Indeed, at the mo-
ment our system only features basic data encryption
in compliance with the BLE standard, and the MQTT-
based solution does not use any security mechanism.
Security of our overall system needs to be improved,
more particularly in terms of access control (i.e., the
patients of physicians must be able to know and con-
trol who accesses their medical data) and identity
management, and recently developed decentralized
blockchain-based solutions (Zhu et al., 2017) can be
explored in order to provide comprehensive werable
healthcare system security.
5 CONCLUSION
In this paper, we presented a multiparametric, car-
diorespiratory wearable sensor. In order to answer
healthcare requirements, more particularly in terms of
the ability to integrate wearable sensors into wider-
scale frameworks, we considered an IoT-based ap-
proach. Indeed, we equipped our sensor with remote
configuration capabilities while preserving quality-of-
data and real-time streaming capabilities, which are
key requirements of wearable healthcare systems. This
sensor was implemented using carefully selected hard-
ware, and it was comprehensively characterized in
terms of energy consumption, which is another major
concern of wearable healthcare devices. Indeed, be-
cause battery charging usually implies the sensor is
not collecting physiological data, potentially relevant
data can be lost, and the charging time to battery life
ration must thus be as big as possible. The data col-
lection capabilities of our sensor were also extensively
tested on both synthetic ECG signals and in real-life
ambulatory conditions. Successful testing and inte-
gration to Internet-based framework proved that our
sensor can be used in a wide-scale wearable healthcare
framework.
ACKNOWLEDGMENT
The authors would like to thank the COOPERA fund-
ing program of R
´
egion Auvergne Rh
ˆ
one-Alpes for
their generous financial support.
REFERENCES
Altini, M., Polito, S., Penders, J., Kim, H., Van Helleputte,
N., Kim, S., and Yazicioglu, F. (2011). An ECG patch
combining a customized ultra-low-power ECG SoC
with bluetooth low energy for long term ambulatory
monitoring. In Proceedings of the 2nd Conference
on Wireless Health, pages 15:1–15:2, New York, NY,
USA. ACM.
Fernandez, F. and Pallis, G. C. (2014). Opportunities and
challenges of the internet of things for healthcare: Sys-
tems engineering perspective. In Proceedings of the
EAI International Conference on Wireless Mobile Com-
munication and Healthcare, pages 263–266.
Gubbi, J., Buyya, R., Marusic, S., and Palaniswami, M.
(2013). Internet of things (IoT): A vision, architectural
elements, and future directions. Future Generation
Computer Systems, 29(7):1645 – 1660.
Harris III, A. F., Khanna, V., Tuncay, G., Want, R., and
Kravets, R. (2016). Bluetooth low energy in dense
IoT environments. IEEE Communications Magazine,
54(12):30–36.
Izumi, S., Yamashita, K., Nakano, M., Kawaguchi, H.,
Kimura, H., Marumoto, K., Fuchikami, T., Fujimori,
Y., Nakajima, H., Shiga, T., and Yoshimoto, M. (2015).
A wearable healthcare system with a 13.7
µ
A noise tol-
erant ecg processor. IEEE Transactions on Biomedical
Circuits and Systems, 9(5):733–742.
Khayatzadeh, M., Zhang, X., Tan, J., Liew, W. S., and Lian,
Y. (2013). A 0.7-V 17.4-
µ
W 3-lead wireless ECG
SoC. IEEE Transactions on Biomedical Circuits and
Systems, 7(5):583–592.
Lomb, N. R. (1976). Least-squares frequency analysis of
unequally spaced data. Astrophysics and Space Science,
39(2):447–462.
Magno, M., Spagnol, C., Benini, L., and Popovici, E.
(2014). A low power wireless node for contact and
contactless heart monitoring. Microelectronics Journal,
45(12):1656–1664.
Massot, B., Noury, N., Gehin, C., and McAdams, E. (2013).
On designing an ubiquitous sensor network for health
monitoring. In Proceedings of the International Con-
ference on e-Health Networking, Applications and Ser-
vices, pages 310–314.
Massot, B., Risset, T., Michelet, G., and McAdams, E.
(2015). A wireless, low-power, smart sensor of cardiac
activity for clinical remote monitoring. In Proceedings
of the International Conference on E-health Network-
ing, Application Services, pages 488–494.
Massot, B., Risset, T., Michelet, G., and McAdams, E.
(2016). Mixed hardware and software embedded sig-
nal processing methods for in-situ analysis of cardiac
activity. In Proceedings of the 9th International Joint
Conference on Biomedical Engineering Systems and
Technologies, pages 303–310.
Nunan, D., Sandercock, G. R., and Brodie, D. A. (2010).
A quantitative systematic review of normal values
for short-term heart rate variability in healthy adults.
Pacing and Clinical Electrophysiology, 33(11):1407–
1417.
Building IoT-Enabled Wearable Medical Devices: An Application to a Wearable, Multiparametric, Cardiorespiratory Sensor
117

Press, W. H. and Rybicki, G. B. (1989). Fast algorithm
for spectral analysis of unevenly sampled data. The
Astrophysical Journal, 338:277–280.
Sch
¨
afer, A. and Vagedes, J. (2013). How accurate is pulse
rate variability as an estimate of heart rate variability?:
A review on studies comparing photoplethysmographic
technology with an electrocardiogram. International
Journal of Cardiology, 166(1):15 – 29.
Task Force of the European Society of Cardiology the North
American Society of Pacing Electrophysiology (1996).
Heart rate variability: Standards of measurement, phys-
iological interpretation, and clinical use. Circulation,
93(5):1043–1065.
Tuominen, J., Lehtonen, E., Tadi, M. J., Koskinen, J., Pnkl,
M., and Koivisto, T. (2017). A miniaturized low power
biomedical sensor node for clinical research and long
term monitoring of cardiovascular signals. In Proceed-
ings of the International Symposium on Circuits and
Systems, pages 1–4.
Van Hoof, C. and Penders, J. (2013). Addressing the health-
care cost dilemma by managing health instead of man-
aging illness: An opportunity for wearable wireless
sensors. In Proceedings of the Conference on De-
sign, Automation and Test in Europe, pages 1537–1539.
EDA Consortium.
Winter, B. B. and Webster, J. G. (1983). Driven-right-leg
circuit design. IEEE Transactions on Biomedical Engi-
neering, 30(1):62–66.
Zhao, L., Reisman, S., and Findley, T. (1994). Respiration
derived from the electrocardiogram during heart rate
variability studies. In Proceedings of 16th International
Conference of the IEEE Engineering in Medicine and
Biology Society, pages 123–124.
Zhu, X., Badr, Y., Pacheco, J., and Hariri, S. (2017). Auto-
nomic identity framework for the internet of things. In
Proceedings of the International Conference on Cloud
and Autonomic Computing, pages 69–79.
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
118
