
A Simulation-driven Approach in Risk-aware Business Process
Management: A Case Study in Healthcare
Ilaria Angela Amantea, Antonio Di Leva and Emilio Sulis
Computer Science Department, University of Torino, 185 Corso Svizzera, Torino, Italy
Keywords:
Business Process Analysis, Risk Management, Modeling and Simulation, Healthcare.
Abstract:
Risk management in business process is a key factor of success for organization as risks are part of every
business activity. Errors may bring to increased costs, loss of quality as well as time delays, which in healt-
hcare can bring to serious damages. This paper proposes a methodological framework to investigate risks in
organizations by adopting a Business Process Management perspective that includes modeling and simulation
of business processes. We applied our methodology to processes in the Blood Bank department of a large
hospital. Our results show that a simulation-driven approach is an effective way to intercept and estimate real
risks and to provide a decision support to guide the of department’s managers.
1 INTRODUCTION AND
RELATED WORK
One of the main issue of Business Process Manage-
ment (BPM) concerns the analysis of risks related to
a business process and the compliance of the process
to norms, regulations or laws (Dumas et al., 2013; Van
Der Aalst, 2013). Frequently, this forces organizati-
ons in redesign business processes, in the context of
change management (Hayes, 2014).
The most common approach to the issue focu-
ses on the detection of failures, mostly dealing with
bad performance departments, stressing enforcement
styles (Parker and Nielsen, 2011) as well as recon-
sidering project implementations (Hornstein, 2015).
Following a different strategy from common busi-
ness analysis (Chang, 2016), we applied an appro-
ach oriented towards the understanding of cases of
success, as a way to address other departments of the
same organization in process optimization.
Traditional BPM systems usually do not address
the problem of risks that organizations face in their
day-to-day operations. Risk is part of every busi-
ness activity and therefore part of every business pro-
cess. If a risk occurs it may cause loss of quality, in-
creased costs, time delays, complaints and legal pro-
blems (Betz et al., 2011) as well as, in healthcare, se-
rious and permanent damages up to death. So risks
need to be managed and the applications of princi-
ples, frameworks and activities to manage them (com-
monly known as Risk Management) will create soon
a whole range of new regulations. This will lead to
two sets of problems: on one side these regulations
have to be applied so we must pay attention to process
compliance, from the other side new reorganizations
must be implemented with the introduction of new
procedures, i.e. for privacy control. A simulation-
driven approach is a versatile tool to produce results
that are relatively easy to interpret by comparing dif-
ferent scenarios to evaluate process changes (What-if
analysis) (Vom Brocke et al., 2010; Di Leva and Su-
lis, 2017b).
This paper describes a methodology of risk-aware
business process modeling based on process simula-
tion. Our case study refers to Italian “City of Health
and Science” of Torino, one of the biggest hospital in
Europe
1
. In this context, we selected as a use case
a well-performing department (accordingly with the
Risk Manager office of the hospital), the Blood Bank
(BB) department which collects blood or hemocom-
ponents from blood donors and supplies several dif-
ferent hospitals located in the surrounding with blood
products. The department’s laboratory performs tests
necessary for production of blood components (im-
munohematology, blood-borne infectious diseases) as
well as for diagnostic, pre-transfusion testing and pre-
vention of hemolytic disease of the newborn. This pa-
per mainly refers to process modeling techniques used
to analyze and support business processes which in-
volve humans, documents, organizations, or applica-
1
Cfr. Citt
´
a della Salute e della Scienza, http://
www.cittadellasalute.to.it
98
Amantea, I., Leva, A. and Sulis, E.
A Simulation-driven Approach in Risk-aware Business Process Management: A Case Study in Healthcare.
DOI: 10.5220/0006842100980105
In Proceedings of 8th International Conference on Simulation and Modeling Methodologies, Technologies and Applications (SIMULTECH 2018), pages 98-105
ISBN: 978-989-758-323-0
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

tions (Van der Aalst et al., 2010; Di Leva et al., 2017).
Such techniques include specific languages. One of
the most used is the “de facto” standard for process
modeling, the Business Process Modeling and Nota-
tion (BPMN) language (Allweyer, 2016; Smith and
Fingar, 2003). In addition, several tools improve
process analysis allowing the simulation of the pro-
cess itself (Abo-Hamad and Arisha, 2013; Sulis and
Di Leva, 2017). In our case, the iGrafxProcess tool
(iGrafx, 2015) was used to implement the main pha-
ses of the BPM methodology, as well as process simu-
lation and risk analysis. In the context of risk manage-
ment (Sadgrove, 2016; McNeil et al., 2015; Haimes,
2015), most studies investigate specific use cases to
describe benefits of new practices or tools (Tomiyama
et al., 2009; DeRosier et al., 2002; Blake and McTag-
gart, 2016). In healthcare studies this kind of ana-
lysis is particularly important for the direct and indi-
rect consequence of errors (Rose et al., 1992; Vincent
et al., 2000; Fishman, 2013; Chartier, 2014). Some
works focused on evidence based medicine (Vincent
et al., 1998), while others treat favorable cases in pu-
blic health (Braithwaite et al., 2017). In the broad
spectrum of work related to the monitoring of busi-
ness processes, it is possible to find several studies
on compliance with laws, rules or regulations. This
aspect is of particular importance in the case of pro-
cesses related to patient health (Buddle et al., 2005;
Racz et al., 2010; Adams, 2003; Vincent, 2017). In
the following of the paper, we introduce the methodo-
logical framework that has been developed to analyze
and improve business processes (section 2). The ex-
tended process model allows the simulation of actual
(As-is) processes and the execution of What-if analy-
sis of several scenarios which describe possible evo-
lutions (To-be) of these processes. The methodology
also takes into account aspects related to risk analysis
and compliance of processes with current laws and
regulations. Section 3 largely describes the explora-
tion of the case study adopted in this paper: the whole
process that describes the functioning of the hospital
department is reconstructed and its compliance with
current Italian laws and regulations is analyzed. This
analysis is therefore proposed in order to introduce a
new bank management system and analyze the chan-
ges that this hypothesis could have on the existing
process. Finally, some concluding remarks will be
discussed in section 4.
2 THE METHODOLOGICAL
FRAMEWORK
This section introduces our methodological frame-
work that is based on a Risk-aware Business Process
Management (RBPM) methodology (Suriadi et al.,
2014; Jakoubi et al., 2010), including some consi-
derations will be presented on the application of the
methodology in the medical field.
2.1 Risk-aware Business Process
Management methodology
Our methodology consists of three phases:
• Context Analysis: this phase aims to fix the over-
all strategic scenario of the enterprise and to de-
termine the organizational components which will
be investigated.
• Functional Analysis and Process Engineering:
the initial purpose of this phase is the determina-
tion of the activities that are carried out in the cor-
porate functions involved in the process and the
causal relationships existing among them. The
process is then reconstructed starting from exter-
nal input/output events and/or objects: this provi-
des the Process Diagram (sometimes referred to
as a process map or flowchart) that uses the Bu-
siness Process Model and Notation (BPMN) (Al-
lweyer, 2016) specification language to describe
the process. The process model must therefore be
validated with stakeholders involved in the pro-
cess, using animation and simulation of its spe-
cific, obtaining the so called As-is model. This
phase includes the analysis of each cause of er-
rors, such as failures, “near-miss” and behavior
that does not comply with the regulations.
• Risk Analysis and Compliance Verification: the
purpose of this phase is to trace back from the
problems highlighted in the previous phase and to
introduce corrective actions to reduce risks to an
acceptable level or to make activities compliant
with laws and regulations. In this way it is pos-
sible to generate a new version of the As-is mo-
del (the To-be model) which must be verified by
comparing it with the previous version.
2.2 Risk and Compliance Management
in the Medical Field
The Clinical Risk Management (CRM) in hospitals
includes processes, methods, tools and activities used
in handling risks in patient care to increase the safety
of patients and those involved in their care.
A CRM process has to describe the procedure for
handling risk and consists of:
• Risk identification: to perform risk identification
the hospital can take into account notifications
A Simulation-driven Approach in Risk-aware Business Process Management: A Case Study in Healthcare
99

from reporting errors (usually stored into an inci-
dent reporting data base), such as events that cau-
sed problems to patients and complaints. Even re-
sults of inspections and audits can provide useful
indications.
• Risk analysis: the goal of this step is to determine
the causes of risks and factors that favor errors as
well as their effects on the safety of patients.
• Risk assessment: decision-makers must determine
what kind of risks should be treated with priority.
• Risk treatment: a risk can be treated by intro-
ducing preventive measures and/or accepting risk
with or without supervision.
Within a framework of continuous development
the hospital management has to asses the risk mana-
gement system regularly to ascertain whether the risk
handling process achieved the desired goals.
Risk management methods and tools can be pro-
active or reactive. Proactive methods are used in ab-
sence of adverse events while reactive methods are
always preceded by an event. Proactive methods
include Failure Mode and Effect Analysis (FMEA)
(Chiozza and Ponzetti, 2009), Cause and Effect ana-
lysis (“fishbone” diagram) (Nicolini et al., 2011), Sce-
nario analysis (Dumas et al., 2013) and are based on a
systematic data collection. Reactive methods apply a
systematic investigative technique to analyze adverse
events that aims to achieve a comprehensive identifi-
cation of both systemic aspects as well as individual
causes, e.g. London protocol (Vincent et al., 2016).
Compliance refers to the ability of an organization
to comply with the obligations laid down by laws and
regulations. It must become part of the organization
culture and integrate into its processes. Compliance
risk can be characterized by the likelihood of occur-
rence and the consequences of non-compliance with
the organizations obligations.
A compliance framework has to provide the or-
ganizational processes for implementing, monitoring
and continually improving compliance management
throughout the organization. Obviously this kind of
framework is based on detailed knowledge of the data
about errors that occur in the process. For the purpose
of transparency and safety of care, which includes
prevention and management of risk related to the pro-
vision of health services, one of the techniques used
to date and encouraged by most States in the world is
the reporting of adverse events and sentinel events
2
.
2
https://www.jointcommission.org/
sentinel event policy and procedures/
3 THE BLOOD BANK (BB) CASE
STUDY
Blood banking is the process that takes place in the
hospital to make sure that donated blood, or blood
products, are safe before they are used in blood trans-
fusions and other medical procedures. Blood banking
includes typing the blood for transfusion and testing
of infectious diseases. The process begins with the
arrival of a Blood request by using a special form
(we refer to “request” in the rest of the article). In
our case, the BB department consists of three functi-
onal units: Acceptance, Laboratory and Distribution.
In Acceptance requests coming from the other hospi-
tal departments (for example, the Emergency Depart-
ment) are verified: staff should confirm if the infor-
mation on the tube label and on the transfusion re-
quest form are identical. In case of any discrepancy
or doubt, a new sample should be obtained. The re-
quest and the test tube with the patient’s blood is then
sent to the Laboratory. When a patient’s blood sam-
ple arrives at the Laboratory, a certain set of standard
tests are performed, including, but not limited to, the
following:
• Typing: AB0 group (blood type),
• Rh typing (positive or negative antigen),
• Screening for any unexpected red blood cell anti-
bodies that may cause problems in the recipient.
In Distribution, if a unit of blood (or a compo-
nent) is required, it is taken from the blood deposit
and sent to the requesting department through the ap-
propriate staff. In this paper we decided to show only
the Acceptance subprocess for reasons of space, but
the same analysis was carried out for all three (Accep-
tance, Laboratory and Distribution) subprocesses and
full results are provided. We started by reconstructing
the actual Acceptance subprocess as illustrated in Fi-
gure 1. The BPMN language has been used and our
tool allows to insert, after the control gateways, moni-
tors - blocks M1, M2, M3, M4 and M5 - that count all
the transactions that correspond to errors. As shown
in the process diagram, requests are received (Receive
Request), the staff adds requests in the local ma-
nagement system and applies an identifying barcode
(Manage Request). Then checks are carried out on
the correctness of data on the request and the patient’s
blood tube (Check). If errors are detected (moni-
tor M1) the correct data is re-entered. The gateway
Blood components? checks if only blood tests are
necessary or blood components are also required. In
the latter case the doctor of the Blood Bank (BB doc-
tor) verifies the correctness of the request (Evaluate
Request) and, if he has any doubts, calls the doctor
SIMULTECH 2018 - 8th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
100
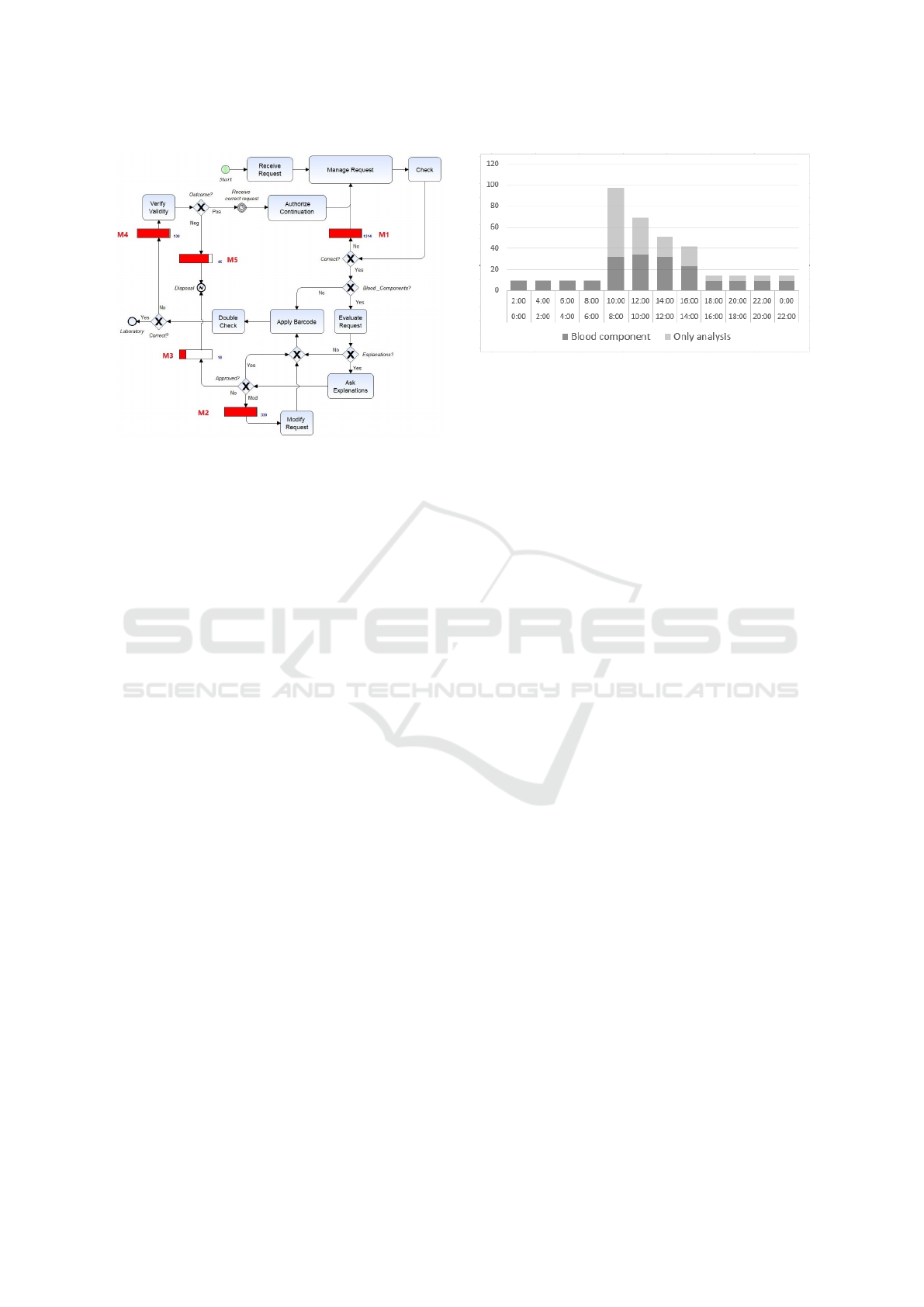
Figure 1: Subprocess of Acceptance of requests with coun-
ters of errors.
of the ordering department for an explanation (Ask
Explanations). At this point (Approved? gateway)
one of three things can happen: 1) the BB doctor is
convinced of the correctness of the request, 2) the re-
quest is changed in agreement between the two doc-
tors (Modify Request), and 3) the request is conside-
red unsuitable and disposed of (the subprocess is clo-
sed with an error report Disposal). For cases 2) and
3) the M2 and M3 monitors count the errors detected
in the request. If no blood components are reque-
sted or the request is deemed suitable or modified, an
identifying barcode is applied on the test tube (Apply
barcode) and a final check is carried out by two pe-
ople together (Double Check), at least one must be a
graduate (doctor or biologist) who eventually puts a
signature of approval on the request. If no errors are
detected (Correct? gateway) the request and the test
tube are sent to the Laboratory, otherwise (Monitor
M4) a check is made with the requesting department
(Verify Validity). Once an agreement is reached, the
requesting department sends a modified request back
with the correct data that is re-entered into the system
(after a certain delay, timer Receive correct request),
otherwise the request is considered unsuitable and de-
leted (monitor M5).
3.1 Process Simulation
In our RBPM methodology the process diagram is in-
tegrated with a description of how each activity deals
with a transaction, how long does it take, and what are
the necessary resources to execute the activity. Furt-
hermore, it is necessary to specify how the transacti-
ons (in our case the requests) are introduced in the
model and for how long the simulation has to last.
Figure 2: The BB daily workload.
The simulation environment is based on the iGrafx
Process tool which is very suitable for process map-
ping and simulation modeling in business process ma-
nagement projects. We perform input data analysis
by considering data about the functioning of the BB
department in 2017. Moreover, we interviewed in-
terviewing physicians, nurses, administrative workers
and managers of he department. The model has been
refined several times and finally validated by workers
and managers of the department.
In particular, for the Acceptance subprocess the
generator that corresponds to the initial event Start
introduces about 350 requests a day distributed accor-
ding to the time table of Figure 2 for a total amount of
about 85,000 requests received from the Blood Bank
during the initial 8 months of 2017. This scenario
has been simulated and, as shown in Table 2, the to-
tal number of errors detected in the subprocess after
8 months of simulation is 1,829 (sum of the M1 - M5
monitors). This number must be compared with the
number of errors reported by the BB staff during the
same period. These errors are stored (together with
the causes that generated them) in a self-reporting da-
tabase (managed by the local system) and shown in
Table 1. In this table the causes of error have been di-
vided according to the units of the Blood Bank where
they can occur, corresponding to Acceptance, Labo-
ratory and Distribution, and the columns TErr, Cau-
ses, Errors and C respectively represent the Type
of Errors, Causes, Errors and Complaints in these
three units. The number of self-reported errors for
the Acceptance is 701. This means that about 62% of
errors has not been self-reported.
For the Laboratory and Distribution units, simi-
lar results are obtained, as shown in Table 2 in which
the columns Rep, Det, DetButNotRep and Com re-
spectively represent the errors Reported, Detected,
Detected but not Reported and the Complaints in the
three units of the Blood Bank and for the whole pro-
cess. The table provides the starting point for two im-
portant conclusions:
A Simulation-driven Approach in Risk-aware Business Process Management: A Case Study in Healthcare
101
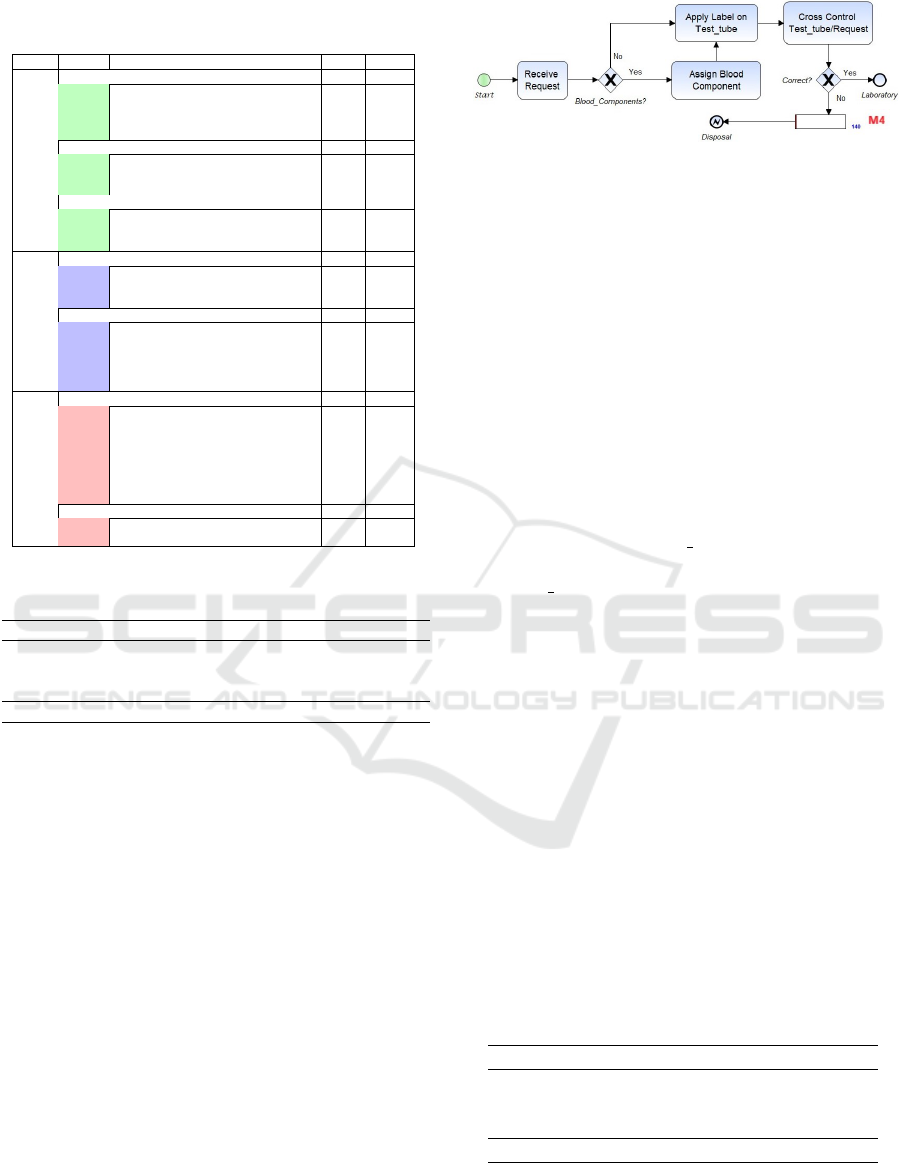
Table 1: Table of reported errors, detected errors and com-
plaints.
Dep TErr Causes Err Com
Acceptance
Internal Acceptance 511 10
Incomplete data 134
Switching Errors 20
Insert Error 349 10
Other 8
Internal Check in Acceptance 127
Cross check (request-test tube) missing 14
Signature check missing 31
Doctor check missing 82
Inappropriate Request 63
Data: inappropriate/reconsidered 16
Quantity: inappropriate/reconsidered 26
Urgency: inappropriate/reconsidered 21
Laboratory
Internal Test 18
Not performed 11
Insert missing 6
Other 1
Internal Assignment 93 4
Barcode check missing 9
Unsuitable reservation 79
Wrong labeling 3
Wrong assignment 1
Computer transmission problem 5
Distribution
Internal Distribution 78 8
Wrong document delivery 1 1
Wrong number of unit delivery 27 1
Late delivery 43
Wrong blood component delivery 1
Error on the medical report 3
No correspondence bag/data 2 5
Various 2
External Output 4
Wrong Department Delivery 2
Switching Errors 2
Table 2: Table of reported errors, detected errors and com-
plaints.
Rep Det DetButNotRep Com
Acceptance 701 1,829 62.0% 10
Laboratory 111 700 84.0% 4
Distribution 78 400 80.5% 12
BB Process 890 2,929 70.0% 26
• The BB staff has a poor attitude for reporting er-
rors as they are discovered in the process. This
is partly due to the workload that at certain times
of the day is particularly heavy. The consequence
of this fact is that the management of the bank has
little information about the actual causes of errors.
As a result, improvement initiatives clearly suffer
from this deficiency.
• The Complaints column shows that the number of
errors not detected in the BB process is very low,
this indicates that the current process is very effi-
cient. In any case, as the consequences of certain
errors can be very serious the need to improve the
process is always present and an FMEA analysis
is under way to address the most dangerous cases.
3.2 Compliance of the BB Process
The efficiency of the BB process is the result of con-
tinuous improvement initiatives. In particular, several
checks on the correctness of data have been introdu-
ced in order to detect the greatest possible number
Figure 3: Acceptance subprocess with only mandatory con-
trols and detected errors.
of errors. It may therefore be instructive to compare,
for example, the current Acceptance sub-process with
what would be if only the rules prescribed by law
were applied. In our case, in the Acceptance subpro-
cess the Italian law only imposes to check that the
surname, name and date of birth of the patient repor-
ted on the request are the same as reported on the test
tube. Figure 3 shows how the subprocess of Accep-
tance would be with only the mandatory controls by
Italian law.
Therefore, at the arrival of the request (Receive
Request) if a blood component is required, it is assig-
ned by the BB doctor (Assign Blood Component). In
both cases, a label identification is applied on the test
tube (Apply Label on Test tube) and then the data on
the request and the test tube is checked (Cross Con-
trol Test tube/Request). If no errors are detected
(Correct? gateway) the request and the test tube are
sent to the Laboratory, otherwise (Monitor M4) the re-
quest is disposed. As shown in Figure 3, only a limi-
ted number (140) of errors would be detected in this
Compliant subprocess (the simulation of the two ver-
sions of the subprocess was performed under the same
conditions). This number must be compared with the
number of errors detected in the current subprocess
(1,829) and this means that about 92% of the errors
would not be detected (lost errors). Table 3 illustrates
the results obtained for the whole BB process if only
mandatory obligations are implemented. In this table
the columns Current, Compliant and Lost respecti-
vely represent the errors detected in the current and
the compliant processes, and the percentage of lost
errors. These results clearly indicate that the controls
required by law are absolutely insufficient.
Table 3: Comparison of current and compliant processes.
Current Compliant Lost
Acceptance 1,829 140 92%
Laboratory 700 53 92%
Distribution 400 43 89%
BB Process 2,929 236 92%
SIMULTECH 2018 - 8th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
102
3.3 Process Improvement
The need to introduce several controls into the Accep-
tance subprocess stems from the fact that the process
relies only on the (hand-filled) paper request which
may contain errors and therefore needs to be chec-
ked several times to ensure that patient and test tube
data are correctly uploaded to the local management
system.
The simulation-based approach in the RBPM met-
hodology is very useful for verifying the possible evo-
lution of the processes currently under investigation
(What-if analysis of possible future scenarios). A
scenario can be considered as a description of a pos-
sible future situation. Scenarios are not meant to be a
complete description of the future, but rather a tool to
consider the basic elements of a possible future and
to draw analysts’ attention to the key factors that can
help to effectively improve the process (Di Leva and
Sulis, 2017a). In the RBPM approach the specifica-
tion of the scenarios to be analyzed is very simple if
they can be defined as changes to be made to the As-is
model.
A new web-based version of the local manage-
ment system is currently under development. The new
system provides for integration with the hospital ma-
nagement system in which the patient and test tube
data will be uploaded by the requesting department.
A simplified paper request and the test tube will then
arrive at the Blood Bank, and both will already have
the correct control barcode inserted. At the same time,
these data will be automatically loaded on the local
system for which the problems related to the local in-
sertion of data are eliminated.
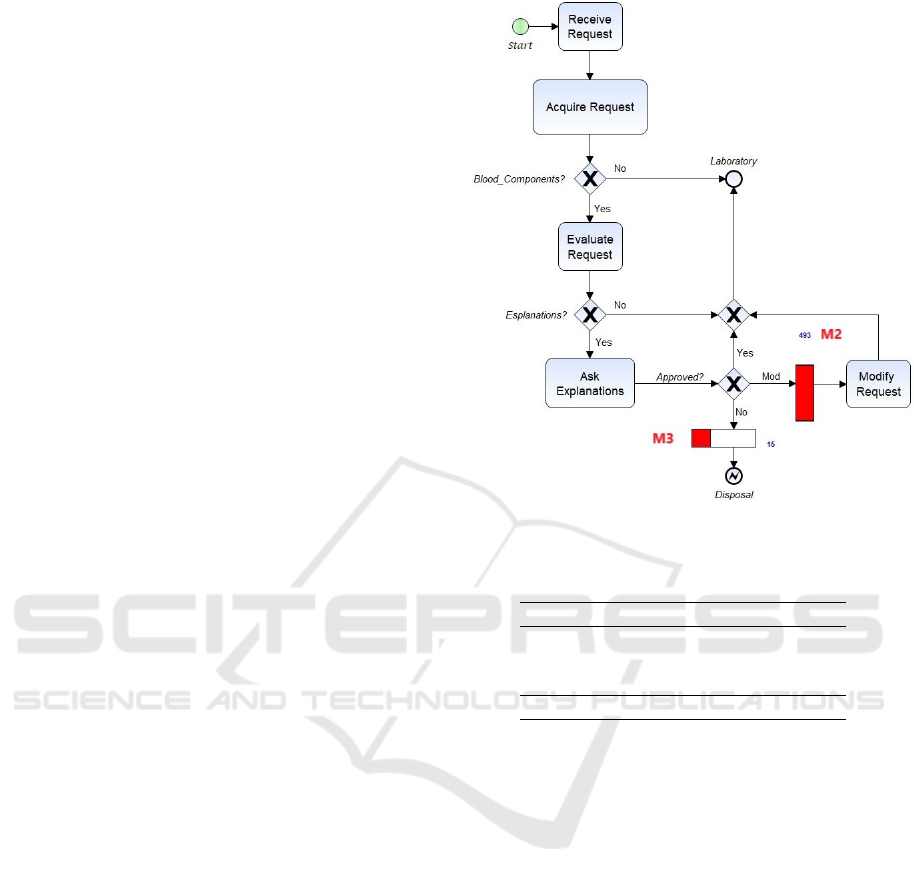
Figure 4 shows how the Acceptance subprocess
could be modified. At the arrival of the request
(Receive Request) the data will be acquired and chec-
ked (Acquire Request) for blood components. If a
blood component is required, as well as for the As-is
model (Figure 1), the BB doctor verifies the correct-
ness of the request (Evaluate Request) and, if he has
any doubts, calls the doctor of the ordering depart-
ment for an explanation (Ask Explanations). At this
point the request could be 1) approved, 2) modified
(Modify Request) or 3) deleted. For cases 2) and 3)
the M2 and M3 monitors count the errors detected in
the request.
The simulation of the two models As-is and To-be
allows a comparison between the two scenarios in re-
lation to the detected errors. Table 4 shows the results
for the whole process. In this table the columns As-
is, To-be and Elim respectively represent the errors
detected by the current As-is subprocess, by the To-
be subprocess and the percentage of errors that would
Figure 4: To-be model of the Acceptance subprocess.
Table 4: Comparison between the As-is and the To-be mo-
del.
As-is To-be Elim
Acceptance 1,829 509 72%
Laboratory 700 494 29%
Distribution 400 335 16%
Process 2,929 1,337 54%
be eliminated from the restructuring. These results
in Table 4 clearly indicate that the introduction of the
new local system greatly reduces (54%) the number
of errors that would be detected in the Blood Bank.
This leads to a much more efficient process in terms
of processing time of requests and costs for the orga-
nization.
4 CONCLUSIONS
In this paper, we described a model-based approach
(RBPM framework) to design and reason about an or-
ganization’s business environment. The framework
includes a methodology to model, validate and ana-
lyze business processes, and an extended process mo-
del that allows the simulation of actual (As-is) proces-
ses and the execution of What-if analysis of scenarios
which describes possible evolutions of these proces-
ses. In this way managers can get useful suggesti-
ons for deciding on the most appropriate restructuring
actions to improve the efficiency of the organization.
A Simulation-driven Approach in Risk-aware Business Process Management: A Case Study in Healthcare
103

The paper illustrates, through a complete case
study, the possibilities offered by the RBPM frame-
work to accurately analyze the effective functioning
of the organization under analysis and to model pos-
sible evolutions towards a more efficient organization.
REFERENCES
Abo-Hamad, W. and Arisha, A. (2013). Simulation-based
framework to improve patient experience in an emer-
gency department. European Journal of Operational
Research, 224(1):154–166.
Adams, A. P. (2003). Clinical risk management. enhancing
patient safety, 2nd edition: Charles vincent (ed.). bmj
books: London, uk, 2001, 573 pp; hardback, indexed,
isbn: 0-7279-1392-1; price 47.50. European Journal
of Anaesthesiology, 20(7):589589.
Allweyer, T. (2016). BPMN 2.0: introduction to the stan-
dard for business process modeling. BoD–Books on
Demand.
Betz, S., Hickl, S., and Oberweis, A. (2011). Risk-aware
business process modeling and simulation using xml
nets. In Commerce and enterprise computing (cec),
2011 IEEE 13th conference on, pages 349–356. IEEE.
Blake, J. and McTaggart, K. (2016). Using simulation for
strategic blood supply chain design in the canadian
prairies. In Simulation and Modeling Methodologies,
Technologies and Applications (SIMULTECH), 2016
6th International Conference on, pages 1–8. IEEE.
Braithwaite, J., Westbrook, J., Coiera, E., Runciman, W.,
Day, R., Hillman, K., and Herkes, J. (2017). A sys-
tems science perspective on the capacity for change in
public hospitals. Israel journal of health policy rese-
arch, 6(1):16.
Buddle, J. J., Burke, B. S., Perkins, R. A., Roday, L. E.,
Tartaglia, R., and Vermiglio, I. A. (2005). System
and method for compliance management. US Patent
6,912,502.
Chang, J. F. (2016). Business process management systems:
strategy and implementation. CRC Press.
Chartier, Y. (2014). Safe management of wastes from
health-care activities. World Health Organization.
Chiozza, M. L. and Ponzetti, C. (2009). Fmea: a model
for reducing medical errors. Clinica Chimica Acta,
404(1):75–78.
DeRosier, J., Stalhandske, E., Bagian, J. P., and Nudell, T.
(2002). Using health care failure mode and effect ana-
lysis: the va national center for patient safetys pro-
spective risk analysis system. The Joint Commission
journal on quality improvement, 28(5):248–267.
Di Leva, A. and Sulis, E. (2017a). A business process met-
hodology to investigate organization management: a
hospital case study. WSEAS Transactions on business
and economics, (14):100–109.
Di Leva, A. and Sulis, E. (2017b). Process analysis for a
hospital emergency department. International Journal
of Economics and Management Systems, 2(1):34–41.
Di Leva, A., Sulis, E., and Vinai, M. (2017). Business pro-
cess analysis and simulation: The contact center of a
public health and social information office. Intelligent
Information Management, 9(05):189.
Dumas, M., Rosa, M. L., Mendling, J., and Reijers, H. A.
(2013). Fundamentals of Business Process Manage-
ment. Springer.
Fishman, G. (2013). Discrete-event simulation: modeling,
programming, and analysis. Springer Science & Bu-
siness Media.
Haimes, Y. Y. (2015). Risk modeling, assessment, and ma-
nagement. John Wiley & Sons.
Hayes, J. (2014). The theory and practice of change mana-
gement. Palgrave Macmillan.
Hornstein, H. A. (2015). The integration of project manage-
ment and organizational change management is now a
necessity. International Journal of Project Manage-
ment, 33(2):291 – 298.
iGrafx (2015). iGrafxProcess 2015. http://www.igrafx.com.
Jakoubi, S., Tjoa, S., Goluch, S., and Kitzler, G.
(2010). Risk-Aware Business Process Management—
Establishing the Link Between Business and Security,
pages 109–135. Springer New York, New York, NY.
McNeil, A. J., Frey, R., and Embrechts, P. (2015). Quan-
titative risk management: Concepts, techniques and
tools. Princeton university press.
Nicolini, D., Waring1, J., and Mengis2, J. (2011). The
challenges of undertaking root cause analysis in he-
alth care: A qualitative study. Journal of Health Ser-
vices Research & Policy, 16(1 suppl):34–41. PMID:
21460348.
Parker, C. and Nielsen, V. L. (2011). Explaining compli-
ance: Business responses to regulation. Edward Elgar
Publishing.
Racz, N., Weippl, E., and Seufert, A. (2010). A process
model for integrated it governance, risk, and compli-
ance management. In Proceedings of the Ninth Bal-
tic Conference on Databases and Information Systems
(DB&IS 2010), pages 155–170. Citeseer.
Rose, G. et al. (1992). The strategy of preventive medicine.
The strategy of preventive medicine.
Sadgrove, K. (2016). The complete guide to business risk
management. Routledge.
Smith, H. and Fingar, P. (2003). Business process manage-
ment: the third wave, volume 1. Meghan-Kiffer Press
Tampa.
Sulis, E. and Di Leva, A. (2017). An agent-based model of
a business process: The use case of a hospital emer-
gency department. In International Conference on Bu-
siness Process Management, pages 124–132. Sprin-
ger.
Suriadi, S., Weiss, B., Winkelmann, A., ter Hofstede, A. H.,
Adams, M., Conforti, R., Fidge, C., Rosa, M. L.,
Ouyang, C., Rosemann, M., Pika, A., and Wynn, M.
(2014). Current research in risk-aware business pro-
cess management : overview, comparison, and gap
analysis. Communications of the Association for In-
formation Systems, 34(1):933–984.
SIMULTECH 2018 - 8th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
104

Tomiyama, T., Gu, P., Jin, Y., Lutters, D., Kind, C., and
Kimura, F. (2009). Design methodologies: Indus-
trial and educational applications. CIRP Annals-
Manufacturing Technology, 58(2):543–565.
Van Der Aalst, W. M. (2013). Business process manage-
ment: a comprehensive survey. ISRN Software Engi-
neering, 2013.
Van der Aalst, W. M., Nakatumba, J., Rozinat, A., and Rus-
sell, N. (2010). Business process simulation. In Hand-
book on BPM 1, pages 313–338. Springer.
Vincent, C. (2017). Patient safety. Wiley-Blackwell.
Vincent, C., Amalberti, R., et al. (2016). Safer healthcare.
Cham: Springer International Publishing.
Vincent, C., Taylor-Adams, S., Chapman, E. J., Hewett, D.,
Prior, S., Strange, P., and Tizzard, A. (2000). How
to investigate and analyse clinical incidents: clinical
risk unit and association of litigation and risk ma-
nagement protocol. BMJ: British Medical Journal,
320(7237):777.
Vincent, C., Taylor-Adams, S., and Stanhope, N. (1998).
Framework for analysing risk and safety in cli-
nical medicine. BMJ: British Medical Journal,
316(7138):1154.
Vom Brocke, J., Rosemann, M., et al. (2010). Handbook on
business process management. Springer.
A Simulation-driven Approach in Risk-aware Business Process Management: A Case Study in Healthcare
105
