
The Influence of Exercise Load and Blood Flow Restriction on the
Recovery of Neuromuscular Strength following Resistance Exercise
Charlie Davids
1,5
, Truls Raastad
3
, Glen Lichtwark
1
, Jeff Coombes
1
, Jonathan Peake
4,5
and Llion Roberts
2,5
1
School of Human Movement and Nutrition Sciences, The University of Queensland, Brisbane, Australia
2
Griffith Sports Physiology and Performance, School of Allied Health Sciences, Griffith University, Southport, Australia
3
Department of Physical Performance, Norwegian School of Sport Sciences, Oslo, Norway
4
School of Biomedical Sciences, Queensland University of Technology, Brisbane, Australia
5
Queensland Academy of Sport, Nathan, Queensland, Australia
1 OBJECTIVES
Due to the diverse demands in many sports, athletes
are required to simultaneously develop multiple
facets of physical fitness. This often requires multiple
training sessions within short timeframes, meaning
recovery between sessions is of fundamental
importance to promote optimal performance during
training. Such effects are only exacerbated in the
competitive season when performance during games
becomes the key priority. However, traditional high
load resistance exercise (HL-RE), which is the
current gold standard for enhancing skeletal muscle
adaptations, is accompanied by high levels of
mechanical stress (Schoenfeld, 2010). Mechanical
stress can impair muscular performance in the hours
and days following training, impacting subsequent
training sessions, and competitive performance
(Doma, 2017). Consequently, many coaches reduce
volume and intensity during the season to mitigate
these mechanical stresses, but this approach may lead
to suboptimal stimuli for skeletal muscle adaptation.
A solution to this issue is the combination of
blood flow restriction with low load resistance
exercise (LL-BFR), which has been demonstrated to
produce significant increases in skeletal muscle
hypertrophy, strength and endurance (Clark, 2011;
Kacin and Strazar, 2011). This is achieved with
reduced mechanical stress, as often loads ranging
from 20-30% of one-repetition maximum (1RM) are
used. Restriction of blood, and ultimately oxygen, to
the exercising muscle results in greater metabolic
stress, which appears to compensate for the lack of
mechanical stress. Despite this change in stimulus
from mechanical to predominantly metabolic, it
appears that LL-BFR is still capable of producing
robust hypertrophic and strength gains that are
comparable to HL-RE, even in athletic populations
(Luebbers et al., 2017). Importantly, these
adaptations seem to occur with much less training
volume load (load x sets x reps), enhancing training
efficiency and minimising stress to connective
tissues. However, less is known of the acute recovery
from LL-BFR, and whether the shift in stimulus (from
mechanical to metabolic) observed with this type of
exercise leads to a hastened recovery of muscle
performance. This possibility is supported by the
absence of muscle damage that has been reported
with LL-BFR (Loenneke, 2014). Knowledge of the
timeline of recovery from LL-BFR is necessary to
understand the exercise-adaptation cycle of this
innovative mode of exercise, so an optimal balance of
maximising adaptations while still allowing sufficient
recovery periods, can be achieved.
The majority of previous studies have assessed
neuromuscular performance immediately following
LL-BFR, which appears to be impaired to a similar
extent as HL-RE (Cook, 2013; Loenneke, 2015).
However, neuromuscular performance needs to be
evaluated further into the post-exercise period to
establish an acute timeline of fatigue and recovery.
Husmann (2017) demonstrated that neuromuscular
performance is significantly impaired immediately
following LL-BFR. However, performance improves
drastically within 8 minutes upon reperfusion of the
exercising muscles, perhaps indicating that acute
strength impairment is a result of peripheral fatigue
caused by metabolite accumulation, as opposed to
central factors. It is important to acknowledge that
strength did not completely recover to pre-exercise
levels. Indeed, Loenneke (2013) reported these levels
were still not regained with LL-BFR at 60 minutes
post-exercise. However, it is not clear how these
effects compare to the use of HL-RE, and whether
there is a difference in the origin of fatigue (be it
Davids, C., Raastad, T., Lichtwark, G., Coombes, J., Peake, J. and Roberts, L.
The Influence of Exercise Load and Blood Flow Restriction on the Recovery of Neuromuscular Strength following Resistance Exercise.
In Extended Abstracts (icSPORTS 2018), pages 19-23
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
19

central or peripheral) observed after both exercise
protocols.
Therefore, the aim of the present study was to
compare how low body neuromuscular performance
was influenced 60 minutes after lower body
resistance exercise is influenced by blood flow
restriction (BFR) protocol type, and exercise load. It
was hypothesised that although the addition of BFR
to low load exercise would enhance strength
decrements following exercise, such effects would
still be reduced in comparison to high load exercise.
2 METHODS
Participants.
Twelve healthy resistance-trained males (mean
standard deviation; age: 22.3 3.2 years; height:
182.1 6.3cm; body mass: 84.1 9.0kg) volunteered
to participate in the study. All participants had been
resistance training continuously for a minimum of
two years leading up to the trials as an attempt to
translate the findings to athletic cohorts. This study
has been approved by the Human Research Ethics
Committee at The University of Queensland.
Experimental Design.
A randomised within-participants repeated measures
experimental design was used to assess the
neuromuscular responses to different BFR protocols
and exercise intensities. After baseline and
familiarisation visits, participants attended the
laboratory on four occasions, separated by a
minimum of 5 days to complete four experimental
trials in a randomised manner. The conditions were:
(a) low load resistance exercise (LL, 30%1RM); (b)
LL with continuous blood flow restriction (LL-
CBFR); (c) LL with intermittent blood flow
restriction (LL-IBFR); (d) high load resistance
exercise (HL, 70%1RM).
Baseline Visits.
Participants first had their arterial occlusion pressure
(AOP) determined via Doppler ultrasound
(uSmart3300, Terason, USA) of the posterior tibial
artery, as previously advocated by Loenneke et al.,
(2015). Participants then completed the baseline
strength testing on the isokinetic dynamometer as
described below. On a separate visit, participants
performed their 1RM squat, and completed a
familiarisation of the LL-CBFR condition, as this has
been demonstrated to be the most challenging
(Brandner and Warmington, 2017).
Experimental Trials.
Each experimental visit began with a lower body
exercise session. The session consisted of 4 sets of
barbell squat exercise, with 2 minutes of seated inter-
set rest in between. For all LL conditions, the first set
consisted of 30 repetitions, following by three sets of
15 repetitions. For the HL condition, 4 sets of 10
repetitions were completed. Following the final set of
exercise, participants remained seated for 60 minutes.
Blood Flow Restriction Protocol.
For both CBFR and IBFR trials, participants had a
pair of 8cm-wide nylon pneumatic cuffs placed
around the proximal thigh. The cuffs were inflated to
60% of the ultrasound determined AOP immediately
prior to the first set of exercise using a rapid cuff
inflator (E20, Hokanson, Bellevue, WA). In the
CBFR trial, the cuffs remained inflated until the final
set of exercise was completed, whereas during the
IBFR trial, the cuffs were deflated following each set,
and re-inflated immediately prior to beginning the
next set.
Neuromuscular Strength Assessment.
During the baseline visit, and 60 minutes following
exercise during each of the experimental trials,
participants completed a series of maximal isometric
contractions on an isokinetic dynamometer (Biodex
3, Biodex Medical Systems, USA). Prior to being
seated in the dynamometer, participants had reusable
stimulation electrodes (50mm x 90mm; Metron,
Patterson, UK) placed over the femoral nerve. The
cathode electrode was placed just below the inguinal
fold on the anterior groin, with the anode electrode
placed underneath the gluteal fold on the posterior
thigh. Participants were then seated with a 55-degree
hip angle, with their dominant leg strapped to the
lever arm of the machine. The lever arm was fixed at
an angle corresponding to 70 degrees of knee flexion
(full knee extension defined as 0 degrees of flexion).
Following three warm-up submaximal voluntary
contractions of the knee extensors, participants
performed three 5 second maximal voluntary
contractions, each separated by 120 seconds.
Participants were instructed to apply force as rapid
and as hard as possible for the entire 5 seconds. The
peak torque value generated during the best
contraction was recorded as the maximal voluntary
torque (MVT). The rate of torque development was
also calculated by determining the time taken to reach
50% (TPT50) and 90% (TPT90) this peak torque
value.
Following these voluntary contractions,
involuntary activation of the knee extensors was
icSPORTS 2018 - 6th International Congress on Sport Sciences Research and Technology Support
20
achieved via supramaximal stimulation of the femoral
nerve through the stimulation electrodes, connected
to a Digitimer DS7AH (Digitimer Ltd, Welwyn
Garden City, Hertfordshire, UK). Participants
performed an additional three maximal voluntary
contractions, during which the knee extensors were
maximally stimulated, with another maximal
stimulation to the resting muscle following
approximately 3 seconds after the contraction.
Utilising the interpolated twitch technique, voluntary
activation of the quadriceps was determined, as well
as evoked twitch torque from the resting stimulation.
Statistical Analysis.
Data were initially checked for normality using a
Shapiro-Wilk test. Repeated measures two-way
ANOVAs were then used to compare differences
between trials and time points (baseline vs 1-hour
post-exercise). Significant main effects of time,
condition, or interaction were followed by post-hoc
repeated measures t-tests, with Bonferroni’s multiple
comparisons correction. Effect sizes (Cohen’s d)
were also calculated to provide magnitude-based
inferences. Effect sizes were assessed as 0.2 = small
effect, 0.5 = moderate effect, and ≥0.8 = large effect.
Statistical significance levels were accepted at
p<0.05.
3 RESULTS
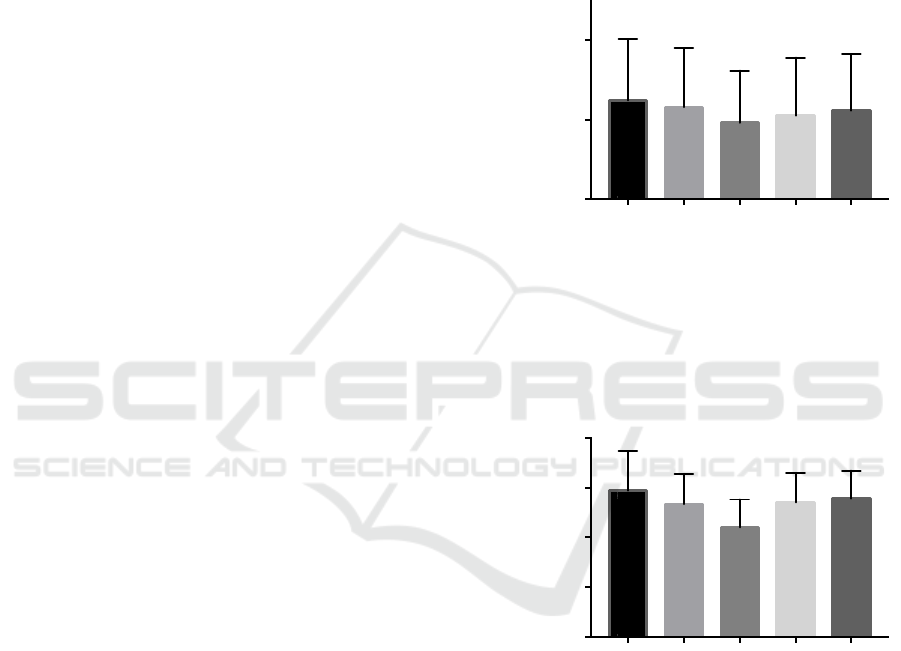
There was a significant time x trial interaction for
MVT (p=0.03). Post hoc analyses revealed significant
time interactions for HL (p<0.01; -8.77%; ES=0.56)
and CBFR conditions (p<0.01; -5.90%; ES=0.36),
while both LL and IBFR showed no significant
change from baseline (Figure 1). Significant
condition interactions were also found between HL
and LL (p<0.01; ES=0.40), and HL and IBFR
conditions (p=0.02; ES=0.23) with no other
interactions between conditions reported (Figure 1A).
There was a significant time x trial interaction for
evoked twitch torque (p<0.01). Post hoc analyses
revealed significant time interactions for HL (p<0.01;
-18.75%; ES=1.51), CBFR (p=0.01; -5.96%;
ES=0.47) and LL (p<0.01; -6.79%; ES=0.53)
conditions, while no change from baseline was
reported for IBFR. Significant trial interactions were
also found between HL and each of the other
conditions (p<0.01 for all, Figure 1B).
No significant change in voluntary activation of
the knee extensors was observed in any of the
conditions (p=0.40; Table 1). There were significant
time interactions for TPT50 with LL, CBFR and
IBFR conditions being higher than baseline, but no
between condition interactions were found (Table 1).
For TPT90, there were significant time interactions
for HL and CBFR, and a significant condition
interaction, with CBFR being significantly different
from LL and IBFR conditions (Table 1).
Figure 1: (A) maximal voluntary torque and (B) evoked
twitch torque of the knee extensors. Black bars represent
baseline values. *indicates a significant difference from
baseline (p<0.05). #indicates a significant difference from
the low load (LL) and intermittent BFR (IBFR) condition
(p<0.05). &indicates significant different all other
conditions (p<0.05).
A
B
Baseli
n
e
L
o
w
Load
Hig
h
L
o
a
d
Co
n
ti
n
u
o
u
s
BFR
I
n
t
erm
i
tte
nt
BFR
200
300
400
Condition
Peak Torque (Nm)
Maximal Voluntary Torque
*
*
#
B
a
se
lin
e
L
ow
Load
H
ig
h
Loa
d
C
ont
inu
ous
B
FR
I
nter
mi
tte
nt
B
FR
20
40
60
80
100
Condition
Twitch Torque (Nm)
Evoked Twitch Torque
*
*
&
*
The Influence of Exercise Load and Blood Flow Restriction on the Recovery of Neuromuscular Strength following Resistance Exercise
21

Table 1: Voluntary activation, time to 50% peak torque (TPT50), and time to 90% peak torque (TPT90). *indicates a
significant difference from baseline (p<0.05). **indicates a significant difference between LL and IBFR conditions.
BAS LL HL CBFR IBFR
Voluntary
Activation (%)
94.425.7 96.633.7 97.942.2 95.844.5 97.213.1
TPT50 (ms) 0.090.01 0.130.06* 0.120.03 0.140.07* 0.150.09*
TPT90 (ms) 0.710.35 0.850.31 0.960.32* 1.100.36** 0.860.27
4 DISCUSSION
The present study compared the decrements in
neuromuscular performance between different lower
body exercise protocols, varying in exercise intensity,
and blood flow restriction application. The primary
findings of the present study indicated that at 60
minutes post-exercise: (i) compared to baseline
levels, MVT was significantly impaired following
only HL and CBFR conditions, whereas there were
no differences from baseline following LL or IBFR
conditions; (ii) compared to baseline levels, evoked
twitch torque was significantly impaired following
HL, CBFR and LL conditions, with no change after
IBFR; and (iii) there were no changes in central
activation of the knee extensors in any of the
conditions compared to baseline levels. These
findings partially supported the hypothesis. Although
both CBFR and HL exercise resulted in significant
neuromuscular performance impairment at 60
minutes post-exercise, there were no significant
differences between conditions.
Previous studies examining the influence of blood
flow restricted exercise on neuromuscular
performance have often assessed this effect
immediately post-exercise. It remains unclear how
performance is recovered acutely in the hours
following exercise. Immediately post-exercise, it
appears that the combination of BFR with low-load
exercise tends to exacerbate the magnitude of fatigue,
and that this effect occurs due to contractile
perturbations caused my metabolite accumulation
(Husmann, 2017). This effect tends to remain at 1
hour following exercise, with strength performance
recovering to baseline levels in the unrestricted
condition (Loenneke, 2015). This outcome aligns
with the results of the present study. Maximal
voluntary torque remained significantly reduced at 60
minutes post-exercise following CBFR, whereas
strength recovered to baseline levels in both LL and
IBFR conditions. It is likely that the larger degree of
metabolic stress experienced in the CBFR condition
caused greater perturbations within the skeletal
muscle, impairing the contractile function.
Interestingly, despite the additional exercise volume
completed in the HL condition (volume-load = load x
sets x reps; HL: 3941.6 485kg; LL, IBFR, CBFR:
3171.9 370kg), MVT remained impaired at 60
minutes post-exercise in both HL and CBFR
conditions, with no differences between them. This
finding suggests that the restriction of oxygen to the
working muscles during exercise and rest periods that
occurs with CBFR, leads to metabolic perturbations
within the skeletal muscle that match those of higher
loads and higher volumes of exercise.
This explanation is further supported by the
reduction in evoked twitch torque, and increase is
TPT90 that was observed in the present study for HL
and CBFR conditions. Evoked twitches consisted of
supramaximal stimulations being delivered to the
knee extensors, meaning the reduction in torque after
exercise is due to factors distal to the neuromuscular
junction. This observation adds weight to the claim
that the fatigue observed in the present study is of
peripheral origin and is related to metabolite
accumulation. Further support for this idea was
provided by Suga (2012), who observed metabolic
stress (indicated by inorganic phosphate
accumulation and pH decline) to increase over the
course of four sets of exercise to match levels seen
with HL exercise. While acute impairments in
neuromuscular performance are not a valid indicator
of chronic hypertrophy, they do tend to align with the
results of chronic studies which report similar
hypertrophy between CBFR and HL conditions, with
inferior hypertrophy in load-matched unrestricted
conditions. This possibility could suggest that CBFR
may be used as a tool to achieve similar hypertrophy
as HL training, despite a marked reduction in training
volume, although chronic training studies are
required for confirmation.
The lack of change in voluntary activation of the
knee extensors found in the present study aligns with
previous findings. While Husmann (2017) found
central activation to be reduced immediately after the
icSPORTS 2018 - 6th International Congress on Sport Sciences Research and Technology Support
22

fourth set of LL-BFR, this effect rapidly recovered
upon reperfusion at 2 minutes post-exercise. Further,
Cook (2013) found no change in central activation
post-exercise between HL, LL-BFR or LL conditions.
This outcome would explain the lack of change seen
at 60 minutes post-exercise in the present study.
Together, with the results mentioned previously,
evidence suggests that the decrement in
neuromuscular performance observed in the present
study is due to peripheral fatigue, as opposed to
central factors.
In conclusion, HL and CBFR squat exercise
appears to impair neuromuscular performance to a
similar extent at 1-hour post-exercise despite the
reduced mechanical stress and total training volume
completed in the CBFR condition. The impairment in
performance was due to peripheral factors as
voluntary activation of the knee extensors remained
unchanged following exercise. Further research
should seek to extend the timeline of neuromuscular
performance recovery past 60 minutes to determine if
differences exist between HL and CBFR.
Furthermore, whether the equivalent acute
neuromuscular responses between HL and CBFR
exercise translate to similar chronic hypertrophic
changes should be evaluated, as LL-BFR training
may serve as a strategy to manage total training stress
and chronic fatigue during busy periods of training
and competition.
ACKNOWLEDGEMENTS
This work is supported by the Queensland Academy
of Sport’s Sport Performance Innovation and
Knowledge Excellence unit.
REFERENCES
Brandner, C. R., & Warmington, S. A. (2017). Delayed
onset muscle soreness and perceived exertion following
blood flow restriction exercise. J Strength Cond Res.
doi:10.1519/jsc.0000000000001779.
Clark, B. C., Manini, T. M., Hoffman, R. L., Williams, P.
S., Guiler, M. K., Knutson, M. J., Kushnick, M. R.
(2011). Relative safety of 4 weeks of blood flow-
restricted resistance exercise in young, healthy adults.
Scand J Med Sci Sports, 21(5), 653-662.
doi:10.1111/j.1600-0838.2010.01100.x.
Cook, S. B., Murphy, B. G., & Labarbera, K. E. (2013).
Neuromuscular function after a bout of low-load blood
flow-restricted exercise. Med Sci Sports Exerc, 45(1),
67-74. doi:10.1249/MSS.0b013e31826c6fa8.
Doma, K., Deakin, G. B., & Bentley, D. J. (2017).
Implications of Impaired Endurance Performance
following Single Bouts of Resistance Training: An
Alternate Concurrent Training Perspective. Sports Med,
47(11), 2187-2200. doi:10.1007/s40279-017-0758-3.
Husmann, F., Mittlmeier, T., Bruhn, S., Zschorlich, V., &
Behrens, M. (2017). Impact of Blood Flow Restriction
Exercise on Muscle Fatigue Development and
Recovery. Med Sci Sports Exerc.
doi:10.1249/mss.0000000000001475.
Kacin, A., & Strazar, K. (2011). Frequent low-load
ischemic resistance exercise to failure enhances muscle
oxygen delivery and endurance capacity. Scand J Med
Sci Sports, 21(6), e231-241. doi:10.1111/j.1600-
0838.2010.01260.x.
Loenneke, J. P., Allen, K. M., Mouser, J. G., Thiebaud, R.
S., Kim, D., Abe, T., & Bemben, M. G. (2015). Blood
flow restriction in the upper and lower limbs is
predicted by limb circumference and systolic blood
pressure. Eur J Appl Physiol, 115(2), 397-405.
doi:10.1007/s00421-014-3030.
Loenneke, J. P., Thiebaud, R. S., & Abe, T. (2014). Does
blood flow restriction result in skeletal muscle damage?
A critical review of available evidence. Scand J Med
Sci Sports, 24(6), e415-422. doi:10.1111/sms.12210.
Loenneke, J. P., Thiebaud, R. S., Fahs, C. A., Rossow, L.
M., Abe, T., & Bemben, M. G. (2013). Blood flow
restriction does not result in prolonged decrements in
torque. Eur J Appl Physiol, 113(4), 923-931.
doi:10.1007/s00421-012-2502.
Luebbers, P. E., Witte, E. V., & Oshel, J. Q. (2017). The
Effects Of Practical Blood Flow Restriction Training
On Adolescent Lower Body Strength. J Strength Cond
Res. doi:10.1519/jsc.0000000000002302.
Schoenfeld, B. J. (2010). The mechanisms of muscle
hypertrophy and their application to resistance training.
J Strength Cond Res, 24(10), 2857-2872.
doi:10.1519/JSC.0b013e3181e840f3.
Suga, T., Okita, K., Takada, S., Omokawa, M., Kadoguchi,
T., Yokota, T., Tsutsui, H. (2012). Effect of multiple set
on intramuscular metabolic stress during low-intensity
resistance exercise with blood flow restriction. Eur J
Appl Physiol, 112(11), 3915-3920.
doi:10.1007/s00421-012-2377-x.
The Influence of Exercise Load and Blood Flow Restriction on the Recovery of Neuromuscular Strength following Resistance Exercise
23
