
Application of TiO2/Bentonite on the Catalytic Pyrolysis of Oil
Sludge
Feifei Wang
1
, Penghui Yang
1
,Xuan Qu
1
, Mingming Du
1
and Chengtun Qu
1,2*
1
College of Chemistry and Chemical Engineering, Xi’an Shiyou University,Xi’an 710065, P. R. China
2
State Key Laboratory of Petrochemical Pollution Control and Treatment,Beijing 102206,P.R.China
Email:xianquct@xsyu.edu.cn
Keywords: Oily Sludge, catalyst (TiO2/bentonite), catalytic pyrolysis
Abstract: Oil sludge is one of the hazardous wastes produced in the processes of petroleum exploitation, refining,
transportation and storage. Implementing the harmless and resource treatment of oily sludge is an urgent
problem to be solved. In this paper, TiO2/bentonite was prepared by sol-gel method, and then was
characterized by scanning electron microscope, X-ray diffraction, Infrared Spectroscopy.The effect of
TiO2/bentonite on improving the yields of oil product was studied. As a result, the oil recovery yield
increased from 86.63%w/w to 95.69%w/w. The oil quality is improved compared with no catalyst after the
TiO2/bentonite was added in the oil sludge.
1 INTRODUCTION
Oily sludge is an oily solid waste which generated
from the oil exploration, transportation, refining, and
oily sewage treatment plants. At present, China
produces about 30 million tons of oil sludge per
year, and hundreds of millions of tons of sludge are
dumped, causing extremely serious pollution to the
soil and groundwater. The main ones are the sludge
and oil sand produced during the petrochemical
industry, which have the characteristics of large
production volume, high oil content, high heavy oil
component, less comprehensive utilization methods,
and difficult processing (Yue and Li, 2010). Oily
sludge generally contains a large amount of benzene,
phenol, hydrazine and other substances, which are
also accompanied by toxic substances and
radioactive elements. If improper treatment of oily
sludge, it will not only pollute the environment,
endanger humans, animals and plants, but also cause
the waste of recyclable resources of the oil sludge
(Liu et al., 2013). Therefore, it is urgent to realize
the resource and harmless treatment of oily sludge.
The common treatment methods for oily sludge
include incineration, conditioning, separation,
solvent extraction, electrochemical treatment, and
pyrolysis. The pyrolysis of the sludge (Wang and
Zou, 2004) as an emerging process technology,
bases on the thermal instability of the organic matter
in the sludge and decomposes the organic matter to
gas, liquid fuel, and carbon under the condition of
the atmosphere without oxygen. Pyrolysis has the
advantages of thorough treatment, better effect of
reducing volume, low secondary pollution, and
energy recovery. It is a treatment method that can
effectively make the sludge resource, reduce, and
harmless (Wang and Zou, 2004). In terms of the role
of mesoporous molecular sieve catalysts in catalytic
cracking of heavy oils , it is proposed that bentonite-
loaded titanium catalysts should be used in the
pyrolysis of oil sludge to explore new ideas for the
influence of pyrolysis temperature, oil recovery rate
and carbon residue during pyrolysis. Yan Li and
other authors have reported:Influencing factors for
catalytic pyrolysis of oily sludge and analysis of
pyrolysis products.
2 EXPERIMENTAL SECTION
2.1 Experimental Reagents
Butyl phthalate. Anhydrous ethanol. Distilled water,
acetic acid. Bentonite. Petroleum ether. The reagents
used were all analytically pure reagents. The
experimental water used was deionized water.
62
Wang, F., Yang, P., Qu, X., Du, M. and Qu, C.
Application of TiO2/Bentonite on the Catalytic Pyrolysis of Oil Sludge.
In Proceedings of the International Workshop on Environment and Geoscience (IWEG 2018), pages 62-67
ISBN: 978-989-758-342-1
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2.2 Preparation of Bentonite Loaded
TiO2 Catalyst
25 mL of anhydrous ethanol and 1mL of butyl
titanate were mixed at room temperature, and stirred
for 1 hour to obtain solution A. 2 mL of glacial
acetic acid was added to 10 mLof absolute ethanol
and mixed thoroughly to form solution B; solution B
was slowly added dropwise to solution A under
magnetic stirring, and solution C was obtained after
homogeneous mixing.2g of bentonite was weighed
into solution C and stirred, then 8 ml of distilled
water was added dropwise, and stirred until the gel.
The gel was washed with deionized water, suction
filtered, dried in an incubator at 80°C, and calcined
at a temperature of 500°C for 3 hours. The resulting
white powder is denoted as TiO
2
/bentonite (1)(
Dong et al., 2013). The amount of butyl titanate is
increased by one times and the other conditions are
unchanged.The resulting white powder is denoted as
TiO
2
/bentonite (2).
2.3 Characterization of Catalyst TiO2 /
Bentonite
Scanning electron microscopy (SEM) with EDAX
; Fourier transform infrared spectroscopy (FTIR)
analysis. The catalyst was subjected to XRD
analysis.
2.4 Catalytic Pyrolysis Experiment of
Oily Sludge
The catalyst and oily sludge were mixed and placed
in a pyrolysis furnace, treated at a certain
temperature for a period of time, the oil recovery
rate was investigated, and the influence of the
catalyst on the pyrolysis was examined. The
experimental conditions were as follows: nitrogen
flow rate 100 mL·min
-1
, temperature 450 ℃ ,
pyrolysis time 4 h, heating rate 10℃·min
-1
, catalyst
TiO
2
/bentonite addition 1%.
The oil recovery rate X is calculated according to
the following formula:
X = W
1
/W × 100% (1
)
In the formula: W
1
is the recovered oil quality, g;
W is the oil quality in the sludge, g.
3 RESULTS AND DISCUSSION
3.1 Features of the Catalyst
a. Bentonite
b. TiO
2
/ bentonite (1)
c.TiO
2
/ bentonite (2)
Figure 1: SEM image of Bentonite and TiO2 /bentonite.
3.1.1 Morphological Features
Figure 1 is a SEM image of the support bentonite,
the catalyst TiO
2
/bentonite (1) and the TiO
2
/bentonite (2).It is shown that TiO
2
/bentonite and
carrier Bentonite have little change in morphology.
But TiO
2
/bentonite is flaky, this feature can
effectively increase the catalytic specific surface
area.Increase the area of contact with oily sludge to
improve the catalytic effect.
Application of TiO2/Bentonite on the Catalytic Pyrolysis of Oil Sludge
63
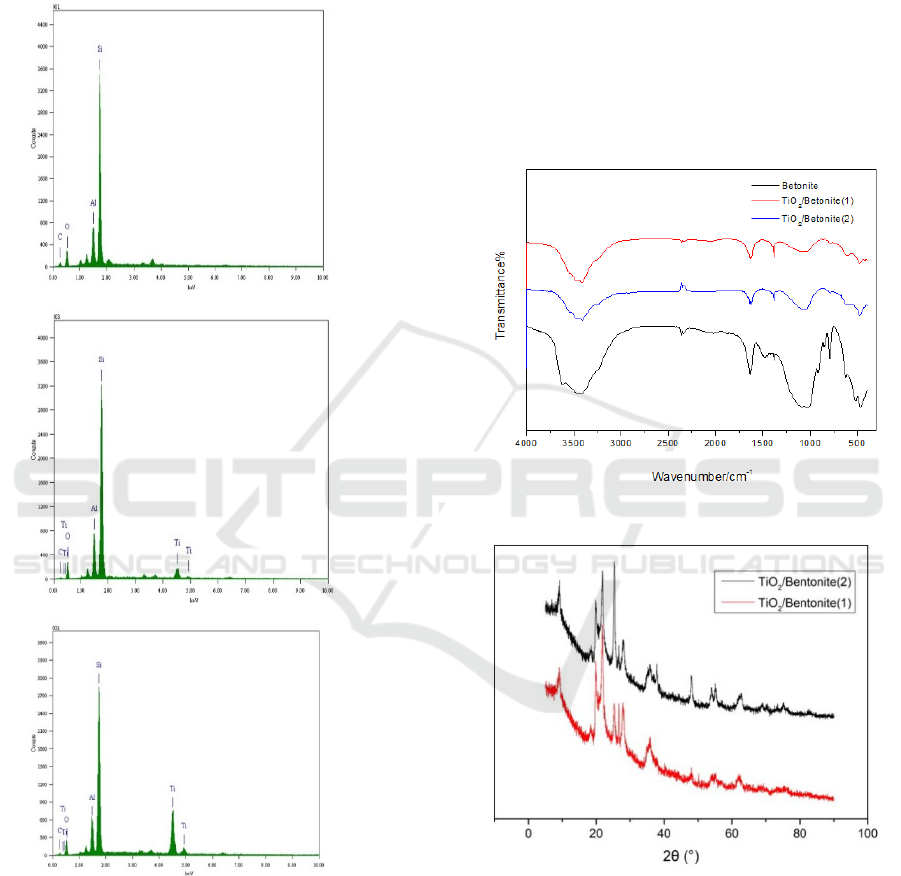
Figure 2 is spectrum elemental analysis diagram
of bentonite, TiO
2
/bentonite (1), and TiO
2
/bentonite
(2).We can see that the samples contain Ti, which
indicates that TiO
2
was successfully supported on
bentonite. In addition, the relative peak value of Si
decreases with the increase of titanium oxide load.
a. bentonit
b. TiO
2
/ bentonite (1)
C.TiO
2
/ bentonite (2)
Figure 2: EDAX of Bentonit and TiO2 /bentonit.
3.1.2 Infrared Spectral Analysis
The infrared spectrum of supported and unsupported
bentonite is shown in Figure 3. It can be seen the
OH stretching vibration of Si-OH-Al in
montmorillonite structure nearby 3630 cm
-1
. It also
can be seen the O-H stretching vibration of adsorbed
water in the interlayer of montmorillonite structure
nearby 3439 cm
-1
. It had stretching vibration band of
O-H near 1642 cm
-1
, and the peak near 1035 cm
-1
is
attributed to the asymmetry bending stretching of Si-
O-Si . The bending vibration absorption peak of Si-
O-Al near 519 cm
-1
, the peak near 1407 cm
-1
is
attributable to the bending stretching of Si-O-Si.
After supported in IR, the peak near 500-700 cm
-1
is
attributable to the bending stretching of Ti-O. The
results revealed that TiO
2
were inserted into
bentonite's layers (Wang et al., 2015).
Figure 3: IR spectrums of bentonite and TiO2 /bentonite.
Figure 4: XRD of TiO2/bentonite.
3.1.3 X-ray diffraction Pattern
Characteristics
A wide-angle XRD spectra of TiO
2
/bentonite (1) and
TiO
2
/bentonite (2) is shown in Figure 4. The anatase
phase of the corresponding diffraction peaks ( 101) ,
( 004) , ( 200) , ( 105) , ( 211) appeared at 2θ = 25.
2°, 37. 8°, 48. 1°, 54. 0° and 55. 2° in both samples.
IWEG 2018 - International Workshop on Environment and Geoscience
64
Rutile phase diffraction peak does not appear.
Shown that the sample has only an anatase phase ,
without rutile phase (Zhang et al., 2014).
TiO
2
/bentonite (1) and TiO
2
/bentonite (2) were
characterized by XRD and the interlayer distance of
them obviously increased. It can be seen from the
figure that the interlayer spacing of TiO
2
/bentonite
(2) is larger than that of TiO
2
/bentonite (1), and the
number of diffraction peaks is increasing. The
results revealed that TiO
2
were inserted into
bentonite's layers.
3.2 Study on Factors Affecting
Pyrolysis of Oily Sludge
3.2.1 Effect of Catalyst Types on the Effect
of Pyrolysis Treatment
The pyrolysis experiments were performed on the
two catalysts at an addition of 1%. The results are
shown in Table 1.
Table 1: Effect of catalyst type
a
.
Catalyst type No
catalyst
TiO
2
/
bentonite
(1)
TiO
2
/bentonite
(2)
Nitrogen flow rate /
(mL·min
-1
)
100 100 100
Temperature
/
℃
450 450 450
Pyrolysis time/h 4 4 4
Heating rate / (℃·
min
-1
)
10 10 10
Oily sludge quality/g 20.07 20.03 20.05
Recovered oil/g 2.85 3.08 3.15
Oil recovery yield/% 86.63 93.90 95.74
Effect of catalyst types on pyrolysis treatment
It is shown from table 1 that the recovery rate of
pyrolysis sludge without catalyst is lower than that
of TiO
2
/bentonite (1) and TiO
2
/bentonite (2). And
the catalytic effect of TiO
2
/bentonite (2) is more
excellent, this is because not only is the titanium
content in TiO
2
/bentonite (2) higher than
TiO
2
/bentonite (1), but also TiO
2
/bentonite (2) is
larger than the surface of TiO
2
/bentonite (1),
increased the contact area of the reaction. This is
consistent with the results of the previous analysis.
3.2.2 Effect of Catalyst Addition on
Thermal Treatment Effect
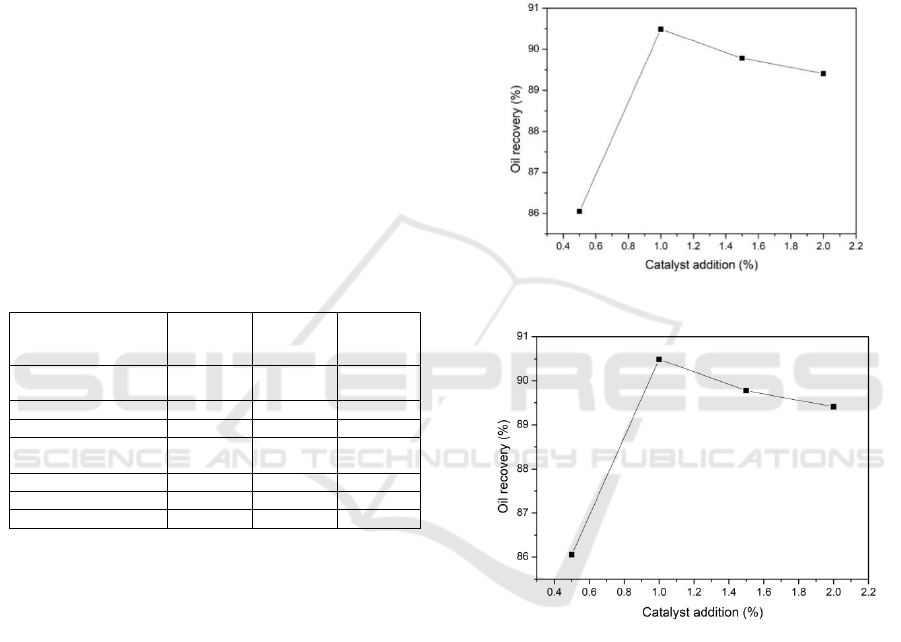
From Figure 5, we can see that the oil recovery rate
increases with the increase of catalyst addition
amount when the catalyst addition is between 0.5%-
1.0%, and it increases from 86.05% to 90.49%;
When the amounts of catalyzer are more than 1%,
the increase of catalyst has little effect on oil
recovery. This is mainly because the oil content of
oily sludge is low, when the amounts of catalyzer
1%, oil recovery rate has reached over 90%, the
activity of the catalyst has been fully played.
Considering the cost of the catalyst, when the
optimum amounts of catalyzer 1%, the oil recovery
rate is the highest.
Figure 5: Effect of catalyst dosage on oil recovery yield.
Figure 6: Effect of pyrolysis temperature on oil recovery
yield.
3.2.3 Influence of Pyrolysis Temperature on
Pyrolysis Treatment
The pyrolysis time was 4 h, the nitrogen flow was
100 mL/min, and the amount of catalyst added was
1%. The effect of temperature on pyrolysis of oil-
containing sludge was investigated. Figure 6 shows
the results.
From Figure 6, we can see that the oil recovery
rate increases obviously with the increase of reaction
temperature when the temperature between 400-420
℃, and it increases from 80.40% to 94.14%, but the
recovery rate of sludge pyrolysis oil decreased when
Application of TiO2/Bentonite on the Catalytic Pyrolysis of Oil Sludge
65
the temperature over 420℃, Because the oily sludge
does not occur pyrolysis reaction at lower
temperatures, when the temperature gradually
increased, macromolecular organic compounds
began to pyrolysis into some small molecules, oil
recovery rate gradually increased, as the temperature
continues to rise, macromolecular pyrolysis reaction
process with many intermediate products will occur
secondary pyrolysis (Li et al., 2006), this results in a
positive proportion of gas production and a gradual
decrease in oil recovery rates. When the temperature
is 420 ℃,and the recovery rate is 10% higher than
that without catalyst. It is indicated that the catalyst
has a strong catalytic effect on the pyrolysis process.
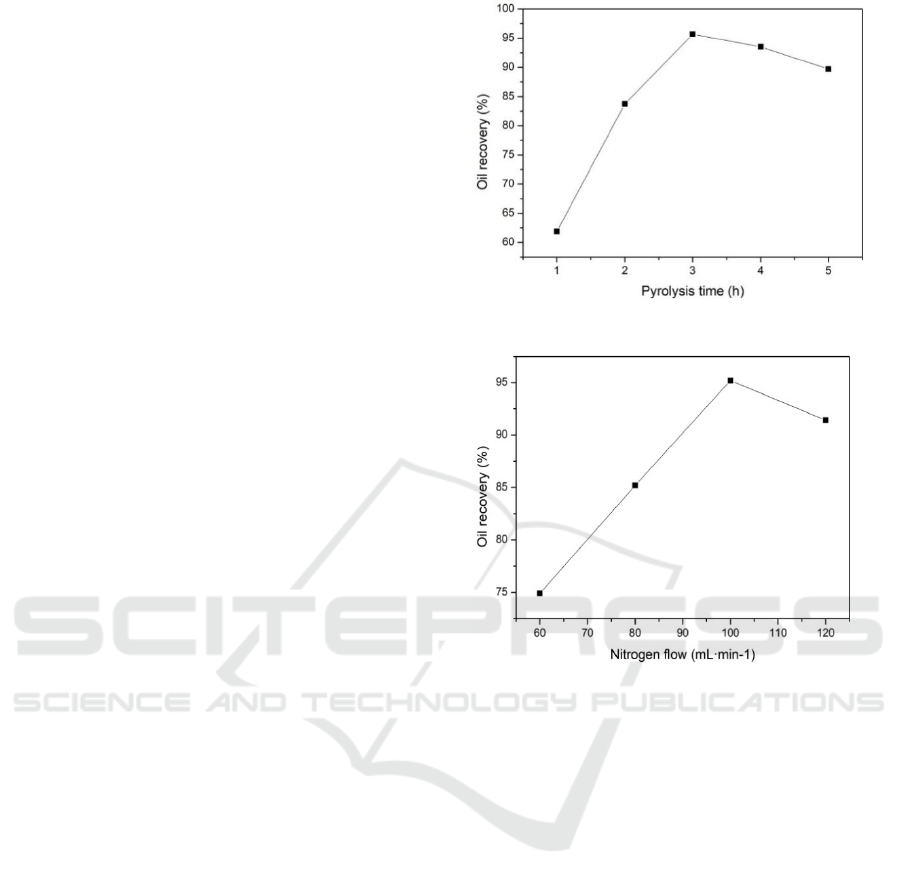
3.2.4 Effect of Time on Catalytic Pyrolysis
of Oily Sludge
From figure 7, we can see in the process of sludge
pyrolysis, the oil recovery rate increases gradually
with the prolongation of residence time when the
reaction time is between 1-3h, and the recovery rate
increased from 61.9% to 95.69%.When the reaction
time is between 4-5h, the oil recovery rate is
decreasing. The reason is that the pyrolysis reaction
is a parallel reaction, the different depth of the
pyrolysis reaction has a great effect on the
distribution of product yield, which will increase the
residence time of the first reaction product in the
pyrolysis reactor, accelerate the secondary reaction
rates, and increase the gaseous phase products
produced by pyrolysis and the solid products
produced by condensation, it weakens the effect of
reaction time on liquid yield and reaction
conversion. It can also be seen that during the
reaction, when the reaction stays for a short time,
some of the sludge has not reached the current
temperature of the full state of pyrolysis, and when
the reaction time is too long, the oil occurred the
secondary pyrolysis (Li et al., 2006), the formation
of gas to discharge, the collection of oil production
reduced and the recovery rate of the reduction.
Therefore, in order to obtain the higher efficiency
and the shorter processing time, the best reaction
residence time is 3h, The pyrolysis time of catalyst
is 1h shorter than that without catalyst. The
experimental results show that the reaction time is
greatly reduced by adding the catalyst, which
reduces the load of equipment and the energy
required for the experiment.
Figure 7: Effect of pyrolysis time on oil recovery yield.
Figure 8: Effect of nitrogen flow rate on oil recovery
yield.
3.2.5 Effect of Nitrogen Flow Rate on the
Results of Pyrolysis Treatment
At the pyrolysis temperature of 420°C and the
pyrolysis time of 3 h, the effect of nitrogen flow rate
on the pyrolysis of oily sludge was investigated.
Figure 8 shows the results.
It can be concluded that the oil recovery rate
increases with the increase of nitrogen flow rate.
Also, it can reach the maximum value when the
nitrogen flow reaches up to 100 mL/min, but it
would decrease when nitrogen flow increased.
Because the oil and gas products in the pyrolysis
furnace can not be purge out in time when the
nitrogen flow rate is too small, causing the
secondary pyrolysis in the furnace and the oil
recovery rate is lower; there is little no-condensing
products that were purge out in the excessive
Nitrogen flow, resulting in decreasing of oil
recovery yield (Zhao, 1985; Li et al., 2018).
IWEG 2018 - International Workshop on Environment and Geoscience
66

4 CONCLUSIONS
The TiO
2
/bentonite supported catalysts were
successfully prepared, and the catalytic effect was
better with the increase of TiO
2
content. When the
pyrolysis temperature is 420 ℃, the pyrolysis time is
3 h, and the nitrogen flow rate is 100 mL/min, the oil
recovery rate can reach 95.69% by adding the
catalyst, and the addition of the catalyst can improve
the oil quality.
REFERENCES
Chen Shuang, Guo Qingjie, Wang Zhiqi and Liu Huie
2007 Study on pyrolysis kinetics of oily sludge
Journal of China University of Petroleum(Edition of
Natural Science) 04 116-120
Dong Yeshuo, Fei Xuening, Jiang Yuanguang, Xie Liping
and Chen Lei 2013 Effect of calcination stage on the
activity of zeolite-supported TiO
2
catalysts Water
Treatment Technology 39 (12) 41-45
Li Haiying, Zhang Shuting and Zhao Xinhua 2006
Influence of pyrogenation temperture of sewage
sludge on product distribution Solar Energy Journal
08 835-840
Li Yan, Hu Haijie Qu Chengtun and Yu Tao 2018
Influencing factors for catalytic pyrolysis of oily
sludge andanalysis of pyrolysis products Modern
Chemical Industry 38(01) 67-71
Liu Fan, Qu Chengtun, Yang Penghui, Xue Jingli and
Yang Wenjuan 2013 Research progress and prospect
of oily sludge treatment technology at internal and
foreign Liaoning Chemical Industry 42 (08) 999-1002
Wang Qiong and Zou Peng 2004 Pyrolysis treatment of
sewage sludge Renewable Resources Research 04 38-
41
Wang Shanshan, Ma Hong zhu, Wang Jing and Yu Jie
2015 Study on modification of active white soil and its
adsorption of phenol wastewater Silicate Bulletin 34
(01) 84-89
Yue Haipeng and Li Song 2010 Development status,
discussion and prospect of oily sludge treatment
technology in oil field Chemical Technology and
Development 04 17-20
Zhang Guangxin, Wang Bing, Zheng Shuilin and Song
Bing 2014 Effect of H
2
O/HAc on the crystal phase and
properties of TiO
2
Nano-Tio
2
/diatomite composites
Journal of synthetic Crystals 43 (05) 1162-1167
Zhao Guang Mu Free radical reaction Beijing: Higher
Education Press 1985
Application of TiO2/Bentonite on the Catalytic Pyrolysis of Oil Sludge
67
