
The Study of Photolysis of Single Nonylphenol Isomer
Min Liu
1,2
, Li Lin
1,2
, Haiyang Jin
1,2
,
Zhuo Huang
1,2
, Liangyuan Zhao
1,2
, Xiaohuan Cao
1,2
, Caixiang
Zhang
3
*
and Xiaoping Liao
3
1
B
asin Water Environmental Research Department, Yangtze River Scientific Research Institute, Wuhan430010, PR China;
2
Key Lab of Basin Water Resource and Eco-environmental Science in Hubei Province, Yangtze River Scientific Research
Institute, Wuhan 430010, PR China;
3
State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences, Wuhan 430074, PR
China.
Email: caixiangzhang@yahoo. com.
Keywords: Photolysis behaviour, photolysis rate, nonylphenol isomer, identified products, pathway
Abstract: To better understand the photolysis behavior of technical nonylphenol (tNP) under low-pressure mercury
lamp (20 W) with light source (λ=253.7 nm), the study of the photolysis of single nonylphenol isomer
(NP
38
) and tNP were carried out. The result showed that NP
38
and tNP were removed quickly, the photolysis
rate of tNP was higher than that of NP
38
. H
2
O
2
accelerated remarkably the degradation of NP
38
. Identified
products of photolysis of NP
38
were probably 4 (2,6-dimethyl-2-heptyl)-1,2-benzenediol or 2 (2,6-dimethyl-
2-heptyl)-1,5-benzenediol, The photolysis of NP
38
seemed to proceed through two pathway: 4 (2,6-
dimethyl-2-heptyl)-1,2-benzenediol was generated by the reaction of NP
38
and O
2
· radicals, the reaction of
NP
38
and ·OH radicals that produced by the photolysis of H
2
O maybe the precursor of 2 (2,6-dimethyl-2-
heptyl) -1, 5-benzenediol, but the H
2
O
2
oxidised the intermediate products could not be detected by GC-MS.
1 INTRODUCTION
Nonylphenol (tNP) was one of endocrine disruptors
(EDCs) due to its estrogenic effect, which was
produced by the degradation of the nonionic
surfactant (NPEO). tNP was more stable, toxic and
accumulate than the NPEO, which was stable in
water (Kannan et al., 2003; Xia et a1., 2013) and had
a significant toxicity effect on zebrafish embryos at
2 µg·L-1 (Zhang et al., 2017). tNP was found in
sewage sludge during the 80s (Giger et al., 1984 ),
and a large amount of tNP were detected on surface
water, groundwater, soil, sediment, air and so on
(Guenther et al., 2002; Liu et al., 2013; Careghini et
al., 2015; Chen et al., 2013; Peng et al., 2016). tNP
was included in the European Union water
framework directive as a new hazardous substance.
The tNP levels was limited at 6.6 µg·L-1 in fresh
water and 1.7µg·L-1 in the seawater (Brooke and
Thursby, 2005).
The degradation rate of tNP under the UV was
about 1.3 times higher than that of natural light
source (Neamţu and Frimmel, 2006). The straight
chain of NP (4-n-NP) degradation rate reached 90%,
after 4 h under certain conditions (Martínez et al.,
2013; Li et al., 2012). The study of degradation of
tNP showed that hydroxyl free (·OH) could
promoted the degradation of tNP under the266 nm
laser flash photolysis and 254 nm photolysis (Zhang
et al., 2012). The above researchers mainly studied
the photolysis of nonylphenol with tNP or 4-n-NP as
the research object, but tNP was composed of a
variety of nonylphenol monomers, and the para
nonylphenol (4-NPs) was mainly composed of alkyl
side chains, which accounted for 86~94%
(Eganhouse et al., 2009). Some study showed that
the photolysis products of the tNP was 4-nonyl-
catechol (Li et al., 2012), but others showed that the
photolysis products of the tNP might be phenols,
aldehydes and carboxylic acids. Therefore, further
research was needed for the photolysis mechanism
of nonylphenol. NP38 monomer was one of 4-NPs,
which was common in the tNP (Eganhouse et al.,
2009; Shan et al., 2011). In this study, NP38 was
chosen as the research object, and its photolysis
82
Liu, M., Lin, L., Jin, H., Huang, Z., Zhao, L., Cao, X., Zhang, C. and Liao, X.
The Study of Photolysis of Single Nonylphenol Isomer.
In Proceedings of the International Workshop on Environment and Geoscience (IWEG 2018), pages 82-86
ISBN: 978-989-758-342-1
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
pathway was discussed, which provided a theoretical
basis for the final fate of tNP in water environment.
2 MATERIALS AND METHODS
2.1 Materials
tNP was bought from Fluka with 99.9% (Germany),
NP
38
(purity, >99%) was synthesized by friedel-
crafts alkylation with 2, 6-dimethyl-2-hepty and
phenol in laboratory (Vinken et al., 2002),
Methanol, n-hexane and Dichloromethane were
chromatographic grade (CNW, Germany). Ultrapure
water (18.2 MΩ·cm) produced by a Mili-Q system,
200 mg·L
-1
stock solutions of NP
38
was prepared
with methanol, the solutions were prepare by adding
an appropriate of stock solution in methanol with
ultrapure water to obtain final concentration 5 mg·L
-
1
of NP
38
.
2.2 Experiment Design
Photolysis batch experiments in water: each
photolysis experiment had two replicates. The
irradiation device with quartz test tubes (volume: 35
cm
3
, average optical path length: 6 cm) in this study
referred to our previous work (Jia et al., 2009). In
brief, the experiment was carried out by using low-
pressure mercury lamp (20 W) as light source
(λ=253.7 nm).Those samples that were wrapped by
the aluminum toil and kept in the dark set as control
group. All Samples were taken at certain time
intervals during the irradiation.
2.3 Extraction and Analysis
tNP and NP
38
determination in water: the water
samples were extracted by liquid-liquid extraction
method. 10 ml of sample was transferred to
separating funnel and extracted three times with 10
ml of DCM, the collected samples were enriched to
about 0.5 ml by a rotary evaporator (Buchi,
Switzerland) and transferred into 2 ml of blown
bottle. They were dried under a gentle nitrogen
stream and redissolved in 0.4 ml of methanol to
being detected by High Performance Liquid
Chromatography (HPLC, 1100-UVD, Agilent
Technologies, USA) (Jia et al., 2009), the crude
products of NP38 were identified by Gas
Chromatograph-Mass Spectrometer (GC-MS, 6890-
5975N, Agilent Technologies, USA) (Shan et al.,
2011).
3 THE RESULTS AND
DISCUSSION
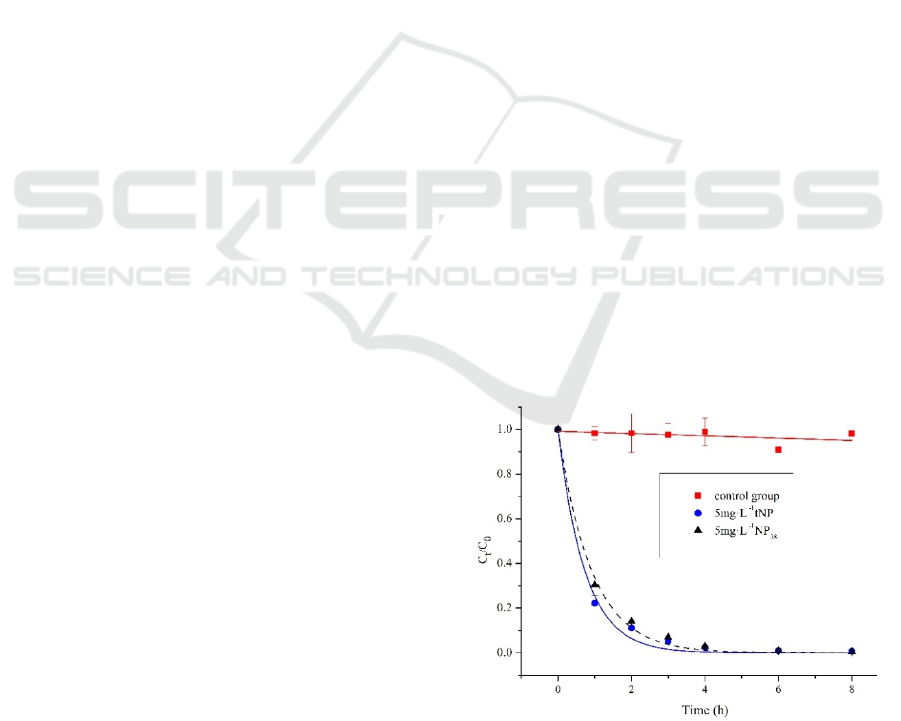
3.1 The Photolysis tNP and NP
38
In the text, Figure.1 shows that tNP and NP
38
can be
efficiently degradated by 78.36% and 69.61%
respectively in an hour through UV photolysis, it
was similar to the degradation of octylphenol (4-OP)
under low pressure mercury lamp (Liao et al., 2009).
The photolysis rate of tNP was fast than NP
38
, due to
the presence of more isomers in tNP that was easy to
degradation than NP
38
. The decay of the tNP in our
study was prominently faster than reported under the
simulate sunlight (Li et al., 2012), which proved UV
light could efficiently decompose tNP.
3.2 The Effect of H
2
O
2
to the
Photolysis of NP
38
Figure.2 shows that only 6.4% and 4.3% of NP
38
existed in the experimental group containing
20mmol·L
-1
and 50mmol·L
-1
H
2
O
2
solution, but the
NP
38
monomer still had 58.2%, after 60 min, in the
solution without H
2
O
2
. This indicates that H
2
O
2
greatly promoted the photolysis process of NP
38
, and
the higher concentration of H
2
O
2
was more
beneficial to the photolysis of NP
38
monomers.
Figure 1: Photolysis of NP
38
and tNP in ultrapure water,
Control group (tNP, 5 mg·L
-1
). Initial condition: pH=6.59,
T=25 ℃, t=8 hours.
The Study of Photolysis of Single Nonylphenol Isomer
83
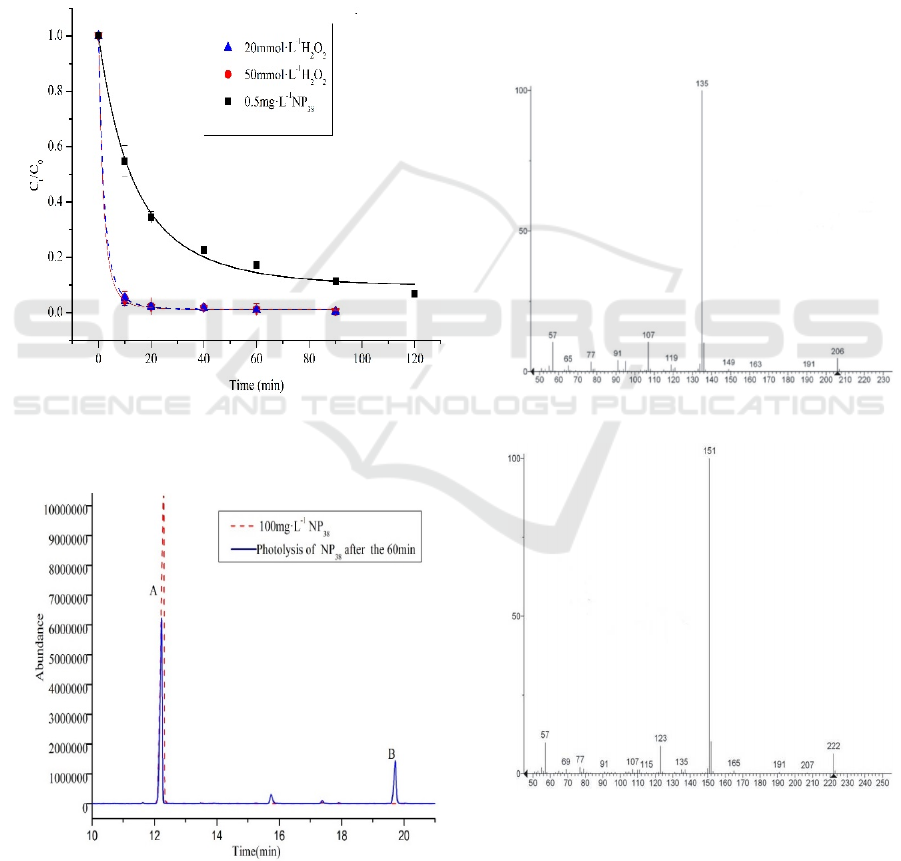
Figure 3 shows that the degradation products of
NP
38
in H
2
O
2
solution were different from those of
NP
38
in ultrapure water system, the total ion
chromatogram (TIC) was shown. A was NP
38
(the
peak time is 12.26 min). B was intermediate
photolysis product (the peak time is 19.72 min), but
it wasn’t detected in the TIC diagram of H
2
O
2
solution, because its intermediate product was
further oxidized to be other substances had different
photolysis pathways.
Figure 2: The effect of H
2
O
2
upon the photolysis of NP
38
.
Initial conditions: C
0
= 0.5 mg·L
-1
, pH= 6.59, T = 25 ℃,
t=120 min.
Figure 3: TIC analysis of NP
38
in ultrapure water after UV
illumination for 60 min.
3.3 The Photolysis Pathways of NP
38
Figure 4 and Figure 5 shows that the MS spectrum
of the NP
38
was mainly included 135 (100), 136
(10), 121 (5), 119 (2), 107 (15) and the MS spectrum
of the B was mainly included 151 (100), 152 (10),
137 (5), 123 (11), 135 (2), because Hydrogen atom
on the benzene ring of the NP
38
was substituted by
hydroxyl group produced B, 135→151, 107→123,
136→152, 121→137, 119→135. 4 (2, 6-dimethyl-2-
heptyl)-1, 2-benzenedio and 2 (2, 6-dimethyl-2-
heptyl)-1, 5-benzenediol was produced by
photolysis of NP
38
( Corvini et al., 2005).
Figure 4: A bar graph of NP
38.
Figure 5: A bar graph of B.
Figure 6 showed two photolysis pathways of
NP
38
. On the one hand, NP
38
was in the excited state
and transferred into the 4-nonyl benzoxy radical
with active ortho hydroxyl group at low-pressure
mercury lamp (20 W) , O
2
·
-
was formed after the O
2
IWEG 2018 - International Workshop on Environment and Geoscience
84
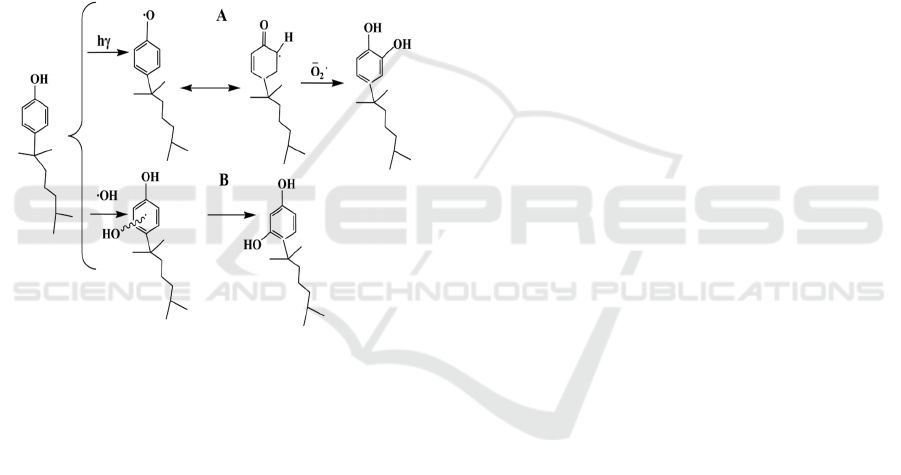
captured electron. The 4 (2, 6-dimethyl-2-heptyl)-1,
2-benzenedio (Figure 6-A) was produced when the
4- nonyl benzoxy radical was attacked by the O
2
·
-
.
On the other hand, hydrogen atoms on adjacent
carbon atoms of NP
38
were more susceptible to be
replaced by the hydroxyl and was converted to 2 (2,
6-dimethyl-2-heptyl)-1, 5-benzenediol (Figure 6-B),
due to the alkyl side chain of NP
38
had the electron
cloud density on the opposite substituent of the
benzene ring (Vinken et al., 2002). There were
different products from 4-n-NP after photolysis (Li
et al., 2012), because of more complex structure of
the alkyl side chain on NP
38
than that of 4-n-NP.
This study indicated that the structure of tNP had a
certain effect on its photolysis behavior.
Figure 6: The possible photolysis pathways of NP
38.
4 CONCLUSIONS
The photolysis behavior of NP
38
was studied under
the conditions of different initial concentration and
different concentration of H
2
O
2
with low pressure
mercury lamp as the light source. the degradation
products of NP
38
were identified by GC-MS, and the
possible degradation pathways of NP
38
were
analyzed. NP
38
and tNP were removed quickly under
the UV radiation. The photolysis rate of tNP was
higher than that of NP
38
. Photolysis would not stop
until all of them disappeared from solution. H
2
O
2
accelerated remarkably the degradation of NP
38
,
NP
38
was degraded about 93.6% and 95.4% in H
2
O
2
20 mmol·L
-1
and 50 mmol·L
-1
. Identified resulting
products were probably 4 (2, 6-dimethyl-2-heptyl) -
1, 2-benzenediol or 2 (2, 6-dimethyl-2-heptyl)-1, 5-
benzenediol. The photolysis of NP
38
seemed to
proceed through two pathway mechanisms: 4 (2, 6-
dimethyl-2-heptyl)-1, 2-benzenediol was generated
by the reaction of NP
38
and O
2
·
-
radicals; The
reaction of NP
38
and ·OH that produced by the
photolysis of H
2
O maybe the precursor of 2 (2, 6-
dimethyl-2-heptyl) -1, 5-benzenediol, but the H
2
O
2
oxidized the intermediate products so that it could
not be detected by GC-MS.
ACKNOWLEDGEMENT
This work was supported by Young Elite
Sponsorship Program by CAST (Grant
2015QNRC001), the National Natural Science
Foundation of China(Grants 51309019), State-level
Public Welfare Scientific Research Institutes Basic
Scientific Research Business Project of China
(CKSF2017062/SH).
REFERENCES
Brooke L and Thursby G 2005. Ambient aquatic life water
quality criteria for nonylphenol. Washington DC,
USA: Report for the United States EPA, Office of
Water, Office of Science and Technology
Careghini A, Mastorgio, A F and Saponaro S, et al. 2015
nonylphenols, benzophenones, and benzotriazoles in
soils, groundwater, surface water, sediments, and food:
a review Environmental Science and Pollution
Research 22 5711
Chen W, Xu J and Lu S, et al. 2013, Fates and transport of
PPCPs in soil receiving reclaimed water irrigation
Chemosphere 93 2621
Corvini PFX, Elend M and Hollender J, et al. 2005
Metabolism of a nonylphenol isomer by sphingomonas
sp. strain TTNP3 Environmental Chemistry Letters 2
185
Eganhouse R P, Pontolillo J and Gaines R B, et al. 2009
Isomer-specific determination of 4-nonylphenols using
comprehensive two dimensional gas chromatography
time of flight mass spectrometry Environmental
Science & Technology 43 9306
Giger W, Brunner P H and Schaffner C 1984 4-
Nonylphenol in sewage-sludge: accumulation of toxic
metabolites from nonionic surfactants Science 225 623
Guenther K, Heinke V and Thiele B, et al. 2002 Endocrine
disrupting nonylphenols are ubiquitous in food
Environmental Science & Technology 36 1676
Jia C Z, Wang Y X and Zhang C X, et al. 2009 Kinetics
study on photocatalytic degradation of bisphenol A
(BPA) by UV/nano-TiO
2
Environmental Pollution &
Control 31 48
The Study of Photolysis of Single Nonylphenol Isomer
85

Kannan K, Keith T L and Naylor C G, et al. 2003
Nonylphenol and nonylphenol ethoxylates in fish,
sediment, and water from the Kalamazoo River,
Michigan Archives of environmental contamination
and toxicology 44 77
Li Y X, Duan X Y and Li X G, et al. 2012 Mechanism
study on photolysis of nonylphenol in water by
intermediate products analysis Acta Chim. Sinica 70
1819
Liao Xiaoping, Zhang Caixiang and Yao Linlin, et al.
2009 Sorption behavior of nonylphenol (NP) on
sewage-irrigated soil: kinetic and thermodynamic
studie Environmental Pollution & Control 31 48
Liu Chunhong, Wang Weihua and Chu Yue, et al. 2013
Dietary exprosure and risk assessment of nonyphenol
and octylphenol from the Shenzhen total diet] China
Environmental Science 33 1316
Martínez-Zapata M, Aristizábal C and Peñuela G 2013
Photolysis of the endocrine-disrupting chemicals 4n-
nonylphenol and triclosan by simulated solar UV
irradiation in aqueous solutions with Fe (III) and in the
absence/presence of humic acids. Journal of
Photochemistry and Photobiology A: Chemistry 251 41
Neamţu M and Frimmel F H 2006 Photolysis of endocrine
disrupting chemical nonylphenol by simulated solar
UV-irradiation Science of The Total Environment 369
295
Peng Duan, Chunhui Hu and Chao Quan, et al. 2016 4-
Nonylphenol induces apoptosis, autophagy and
necrosis in sertoli cells: Involvement of ROS-mediated
AMPK/AKT-mTOR and JNK pathways Toxicology
341-343 28
Shan J, Jiang B and Yu B, et al. 2011 Isomer-specific
degradation of branched and linear 4-nonylphenol
isomers in an oxic soil Environmental Science &
Technology 45 8283
Vinken R, Schmidt B and Schäffer A. 2002 Synthesis of
tertiary14C-labelled nonylphenol isomers Journal of
Labelled Compounds and Radiopharmaceuticals 45
1253
Xia H, WangA and Zhang Y, et a1. 2013 Effects of
nonylphenol on immune function of female Sprague-
Dawleyrats Toxicological
&
Environmental Chemistry
95 658
Zhang Bo, Li Kun and Cao Changqing, et al. 2012 The
laser flash photolysis and UV photolytical degradation
of 4-n-nonylphenol China Science
:
Chemistry 42 175
Zhang Hui, Jiang Jinlin and Zhang YuFeng, et al. 2017
Toxic effects of 4- nonylphenol on zebrafish (danio
rerio) embryo/larva Journal of Ecology and Rural
Environment 33 737
IWEG 2018 - International Workshop on Environment and Geoscience
86
