
Geochemistry of Rare Earth Elements in Sinegorsky Spa of CO
2
-rich
Mineral Water (Sakhalin Island, Far Eastern Russia)
Chelnokov George
1,*
, Bragin Ivan
1
, Kharitonova Natalia
1,2
, Chelnokova Berta
3
, Aseeva Anna
1
and
Bushkareva Kseniya
1
1
Far East Geological Institute FEB RAS, prospect 100-letya 159, 690022,Vladivostok, Russia;
2
Lomonosov Moscow State University, GSP-11, Leninskie Gory, 119991, Moscow, Russia ;
3
Institute of Medical Climatology and Rehabilitation Treatment (Vladivostok branch of FESCRPPR-RIMCRT), Russkaia st.
73-g, 690105, Vladivostok, Russia.
Email: geowater@mail.ru
Keywords: Mineral waters, REE, genesis, pCO
2
waters, mineral precipitates, Sakhalin Island
Abstract: Distribution and abundance of rare earth elements for Sinegorsky Spa of CO
2
-rich mineral waters
and their mineral precipitates on the Sakhalin Island (the Far East of Russia) were studied. The
main common features of waters from six boreholes are Na-Cl-HCO
3
hydrochemical type, high
total dissolved solids (16.2–23.1 g/L), a slightly alkaline pH (6.2– 7.7), and Eh (-181 to 63 mV).
The NASC-normalized patterns of all groundwaters are characterized by HREE enrichments and
positive Eu anomalies, whereas some fluids are characterized by positive Ce anomaly and others
have moderate negative Ce anomaly. The distinct positive Eu/Eu* in mineral waters indicates the
nature of their water-rock interaction whereas Ce anomaly could be the result of the difference in
groundwater circulation. Mineral precipitates are enriched with light REE and show a negative
Eu/Eu* and positive Ce/Ce* anomalies which indicate redox controlled processes. The main
processes controlling dissolved trace element behaviour in water were established. It is argued
that bicarbonate ion-pairs can also play an important role for the solution chemistry of HREE
which explains the significant relative fractionation between REE observed in CO
2
-rich water.
This work was supported by grants from Russian Science Foundation (RSF), p roject № 18-17-
00245.
1 INTRODUCTION
On the Sakhalin Island (the Far East of Russia) the
CO
2
-rich mineral waters are represented by two
major manifestations - Sinegorsky Spa on the south
and Volchansky Spa on the west part of the island.
The first attempt to elucidate the genesis of cold
mineral waters on the Sakhalin Island has been
made in recent years (Chelnokov, et al. 2015;
Chelnokov et al., 2018). It was established that the
studied waters belonged to two main water types: 1)
Na-HCO
3
-Cl alkali carbonate waters with TDS less
than 1.0 g\L and 2) Na-Cl or Na-Cl-HCO
3
saline
groundwaters with TDS up to 26 g/L. The isotopic
data indicate that CO
2
gas in the mineral water may
be mantle-derived and its presence is critical for the
development of the high-pCO
2
groundwater.
The processes controlling the fate and transport
of rare earth elements in fluids are poorly
understood because a complex set of variables
affects rare earth element solubility including
solution chemistry, pH, Eh, solid phase mineralogy
and composition, temperature, and pressure (De et al.
1988; Johannesson et al.1997). For the last years, the
study of the geochemistry of REE in different types
of waters of the Far East of Russia was more active
(Bragin et al. 2016; Chudaev et al. 2016;
Kharitonova et al. 2016) . Presently, we have
indicated new results of REE contents and
distributions in Sinegorsky Spa cold high pCO
2
mineral waters and associated mineral precipitates
located on the Sakhalin Island.
George, C., Ivan, B., Natalia, K., Berta, C., Anna, A. and Kseniya, B.
Geochemistry of Rare Earth Elements in Sinegorsky Spa of CO2-rich Mineral Water (Sakhalin Island, Far Eastern Russia).
In Proceedings of the International Workshop on Environment and Geoscience (IWEG 2018), pages 267-272
ISBN: 978-989-758-342-1
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
267

Table 1: Chemical composition of Sinegorsky high pCO2 mineral waters.
Parameter
Unit
Mineral water boreholes
Fresh GW
№ 963
№ 16
№ 17
№ 18
№ 22
№ 33
№8F
TDS
g/L
16.2
23.1
17.6
17.3
19.3
18.9
0.5
T
o
C
15.3
9.6
10.8
8.6
10.3
9.6
9.5
pH
Unit
6.67
7.4
6.5
6.2
7.1
7
7.7
Eh
mV
-181
69
58
62
-60
-69
63
TOC
mg/L
-
107
27
-
-
-
-
CO2
2000
681
439
650
598
954
-
Na
4780
5612
4528
4282
6978
6298
143
Ca
146.00
270
209
188
245
261.6
13
Mg
91.08
182
114
124
211
221.7
1.46
K
107
29
37
53
59.7
58.17
20.2
Cl
6700
6073
6753
6846
6390
6240
65.2
SO
4
<<
59.6
7.2
7.5
68.2
51.5
1.02
HCO
3
5223
9543
4392
5587
10547
10114
268
Fe
1.0
4.0
1.6
1.6
8.4
8.7
0.3
NH
4
150
95.4
102
106
<<
<<
3
La
ppb
0.0139
0.023
0.010
0.028
0.062
0.063378
0.002
Ce
0.0254
0.032
0.013
0.035
0.152
0.639169
0.000
Pr
0.0013
0.005
0.003
0.007
0.0068
0.009554
0.0004
Nd
0.0169
0.016
0.013
0.029
0.0409
0.036748
0.0014
Sm
0.0114
0.010
0.011
0.017
0.0176
0.021086
0.0009
Eu
0.0501
0.012
0.019
0.018
0.11039
0.121292
0.00356
Gd
0.0131
0.012
0.013
0.018
0.0296
0.048218
0.0010
Tb
0.0016
0.002
0.002
0.002
0.00214
0.002459
0.00008
Dy
0.0084
0.007
0.008
0.010
0.0096
0.010213
0.0003
Ho
0.0016
0.001
0.002
0.002
0.00319
0.001592
0.00010
Er
0.0054
0.006
0.010
0.009
0.01228
0.009914
0.00033
Tm
0.0012
0.002
0.002
0.002
0.00260
0.001244
0.00005
Yb
0.0065
0.009
0.012
0.012
0.01687
0.009282
0.00018
Lu
0.0012
0.002
0.003
0.003
0.00322
0.002851
0.00000
∑REE
0.16
0.14
0.12
0.19
0.47
0.97
0.01
∑LREE
0.13
0.11
0.08
0.15
0.42
0.94
0.01
∑HREE
0.02
0.03
0.04
0.04
0.05
0.04
0.001
Figure 1: Geographic setting of the study area. 1-Sinegorsky spa
high pCO2 mineral water area; 2- boreholes; 3-tectonic dislocations.
IWEG 2018 - International Workshop on Environment and Geoscience
268
The main objective of this study was to analyze
the abundance and distributions of REE in the
aquifer and mineral precipitates of Sinegorsky Spa
(the Sakhalin island) in order to evaluate the
behaviour of REE during water-rock interaction at
low temperature and high CO
2
content.
2 STUDY AREA
Sinegorski spa of a high pCO2 mineral water
located within the southern part of the Central-
Sakhalin fault has been studied. The research objects
are the CO2-rich groundwaters from six boreholes
and mineral precipitates from a borehole № 16
(Figure 1, Table 1). It should be noted, that three
wells are being operated and periodically pumped
(16, 17 and 18) and other three (№ 963, 22 and 33)
are not being used for more than 10 years.
The geological structure of a cretaceous complex
within Sinegorski spa has been studied well in the
previous work (Chelnokov et al. 2018; Niida and
Kito 1986). Boreholes disclose the sandstone aquifer
of Maruyamsky formation of Middle-Pliocene and
Miocene age. The oldest formation in the area is
Mesozoic rocks which consist of micaceous and
quartz shales, marble and quartzite, siltstones,
mudstones, sandstones and tuffs. All sediments of
the cretaceous system had been accumulated in
marine conditions (Chelnokov et al. 2018). The
southern part of the Central-Sakhalin fault is in the
Susunayski artesian basin in terms of hydrogeology.
Hydrogeological conditions of the territory are very
complex and caused by a zone and block structure,
fracture permeability. The zone of an active water
exchange makes not more than 100 m from the
surface and is caused by weathering. Water of the
top aquifers is free-flow, fresh, hydrocarbonate.
Mineral water of the deepest aquifer has
mineralization of 10 - 30 g/l and Cl-Na or HCO
3
-Cl-
Na composition. The associated gases are presented
by CO
2
(50-98 vol.%) and CH
4
(2-45 vol.%)
(Chelnokov et al. 2015)
The boreholes objects of investigations are
located within large tectonic dislocations of the
island. The Sakhalin Island is owed to the Sakhalin-
Hokkaido orogenic belt and is characterized by
tectonic zonality fully represented on the Hokkaido
Island (Japan) and reflects the successive
accumulation of continental crust from the middle
Jurassic to Neogene (Niida and Kito 1986).
3 SAMPLING AND ANALYTICAL
PROCEDURES
The materials obtained by the authors as a result of
their field works carried out in 2015-2017 are used
in the paper. The water samples were filtered
through 0.45 µm mixed cellulose ester filters
(Advantec, Japan) and collected in acid-washed,
high-density polyethylene sample bottles. Waters for
the cation analysis were acidified to pH < 2 with
ultrapure HNO3. Water temperature, conductivity,
and pH were measured directly in the field using
Hach Lange HQ 40D probe. Major cations and
anions were analyzed by the ion chromatography.
Carbonate species were titrated in-situ with 0.1 N
HCl. Trace elements concentrations in groundwater
were determined by ICP-MS (Agilent 7500) analysis.
Trace element and REE concentrations were
analyzed by ICP-MS (Agilent 7500 and ELEMENT
XR) in the Analytical Department of FEGI FEB
RAS (Vladivostok, Russia) and Activation
Laboratories company (Canada, www.actlabs.com).
Analytical precision for the REEs, except for Ce and
Pr, was better than 5% RSD; for Ce and Pr, the
precision was 7% and 10% RSD, respectively. Solid
mineral phase has been investigated in the Far East
Geological Institute, the Far Eastern Branch of the
Russian Academy of Sciences (Primorsky Centre of
Local Elemental and Isotope Analysis). It was
performed using the method of mass-spectrometry
with inductively coupled plasma at the Agilent 7500
spectrometer (the analyst: Elovsky E.V.).
4 RESULTS
The chemical composition of studied waters and
REE contents in water after filtration through filter
0.45 μm are presented in Table 1. Groundwaters are
represented with Na-Cl-HCO3 water type with TDS
varying from 16.2 to 23.1 g/L. All waters are cold,
the pH changes from 6.2 to 7.4 and Eh values vary
from -181 to +63. Na+ dominates as cation species
ranging from 4528 to 6978 mg/L. The
concentrations of Si, Mn, I, Ba are relatively high
(Chelnokov et al. 2018). Local enrichments also
occur in relatively immobile elements such as As
and Sr. While values of Al could be compared with
other analyzed water types. Ammonium
concentrations are remarkably high since organic
matter which is oxidized by sulfate ions in pore
Geochemistry of Rare Earth Elements in Sinegorsky Spa of CO2-rich Mineral Water (Sakhalin Island, Far Eastern Russia)
269
water releases NH4+ and I- ions from entrained
organic matter (e.g., Sholkovitz 1995). The main
sources of the elements in mineral waters are Cl-rich
fluids of a different origin, rock dissolution and
dissolved gases (CO
2
) (Chelnokov et al. 2018). It is
well known that the presence of dissolved CO2
enhances the extent of water-rock interactions,
particularly at the low temperature. Therefore,
associated CO2 gas increases the water-rock
interactions and more intensely leaches elements
from the bedrock. Thermodynamic calculations
indicate that all mineral waters are oversaturated
relative to the primary aluminosilicate minerals
(SIalbite =1.2–2.8), quarts (SIquartz =0.4–1.2),
hydroxides (SIhematite =9.2–17) and carbonate
minerals (SIcalcite =0.2–1.4).
The studied waters had not been analysed
previously with regard to rare earth elements content.
Typical for borehole №33 maximal concentrations
of total REE are almost two times higher than in the
bh № 22 and are remarkably higher than in other
boreholes (Table 1). The minimal concentrations
show fresh groundwaters (bh №8F). To more
conveniently view inter-element trends, the REE
analyses have been normalized to North American
Shale Composite (Gromet et al. 1984). The NASC-
normalized patterns of groundwaters are
characterized by HREE enrichments and positive Eu
anomalies. The interesting difference consists in Ce
anomaly: some fluids are characterized by positive
Ce anomaly (Ce/Ce*=1.1-5.4) (bh № 33, 22, 963)
(Figure 2A) and others have moderate negative Ce
anomaly (Ce/Ce*=0.5-0.6) (bh № 16, 17, 18)
(Figure 2B). Anomalies of Ce were of particular
interest following the ano maly’s potential use as an
indicator of water/rock interaction processes or as a
hydrological tracer (Seto and Akagi 2008).
The positive Eu-anomalies are common for both
groups and are likely reflecting weathering reactions
with host rocks as a result of the preferential
dissolution of a Eu-rich phase (e.g. plagioclase).
Other way groundwater might reflect considerably
more reduced conditions (De et al. 1988; Sholkovit z
1995). Also the Eh conditions of the waters could
specify the difference in Ce anomalies. It is known
that negative Ce anomalies require near-surface
partitioning of Ce due to the oxidation of Ce
3+
to
Ce
4+
and precipitation under oxidative conditions
(Eh>0) (Johannesson et al. 1997; Lewis et al. 1997).
negative Ce anomalies do not occur in reduced
fluids (Eh<0) since Ce
3+
doesn’t oxidize to Ce
4+
.
Also it can be explained by regional bedrock
mineralogy and lithology (Ce-depleted source
minerals in the materials through which the water
flows). Experimental data (Bau, 1999) indicate that
variation in Ce anomaly magnitude could be a result
of the difference in residence time of groundwater
circulation. It is in a good agreement with behavior
of Ce: in boreholes which are being operated (№ 16,
17, 18) we observe Ce minimum and unused
boreholes (№ 963, 22, 33) have Ce maximu m.
The explanation for the HREE-enriched pattern
is that the HREEs form stronger complexes with
ligands in solution than do the LREE. This has two
consequences: the HREEs will be preferentially
released to solution during weathering of source
rocks; and the LREE will be preferentially adsorbed
at particle surfaces in adsorption/equilibria reactions
in waters. The presence of CO
2
gas also enhances
the concentration of heavy REE (Kharitonova et al.
2007). Results of major and trace elements of bulk
mineral precipitates are given in Table 2. The
samples from the top of the well were limpid, white
salt crystals.
The Al
2
O
3
and Na
2
O contents of the sample are
consistently very high (~35%) and (~10%)
correspondingly. Mineral precipitates are enriched
with LREE (89%) compared to the HREE (11%), it
is in good correlation with mineral water producing
this precipitates (Bh №16) containing LREE -79%
and HREE -21% (Tables 1, 2). Comparing the rare
earth element signature of waters with its mineral
precipitates reveals differences (Figure 2). NASC-
normalized concentrations are higher for REE in
solid phase than for the water. The respective
NASC-normalized REE pattern decreases slightly
from La to Nd, increases from Eu to Lu; and shows
a small positive Ce and negative Eu anomalies
(Table 2). Enrichment of Ce in solid phase (Bh №16
Ce/Ce*= 1.2) is clearly indicated by the depletion of
this element in corresponding waters (Bh №16
Ce/Ce*= 0.6). Positive Ce anomaly can indicate
redox controlled processes leading to the formation
of CeO
2
(Aubert et al. 2001). Whereas Eu do not
accumulate in mineral precipitates and demonstrates
lowest level among REE (Table 2, Figure 2B).
IWEG 2018 - International Workshop on Environment and Geoscience
270
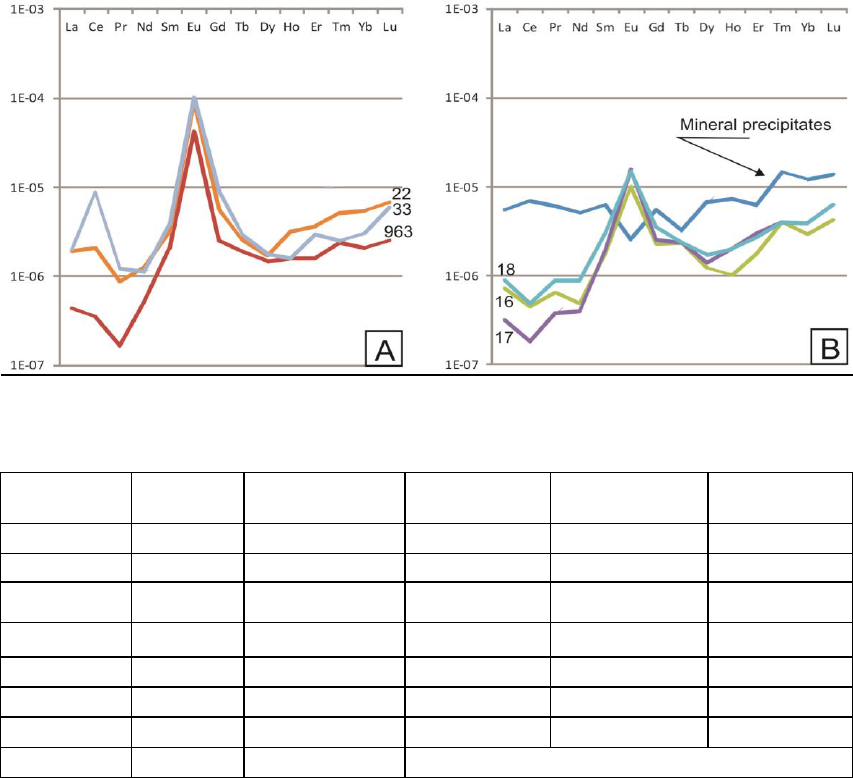
Figure 2: NASC-normalized concentrations of REE in mineral waters and precipitates. А-not operated wells with
positive Ce anomaly; B- operated wells with negative Ce anomaly and their mineral precipitates.
Table 2: Bulk chemical composition of the studied mineral precipitates.
Paramerer
Borehole №
16 [%]
Parameter
Borehole № 16
(ppb)
Parameter
Borehole № 16
(ppb)
SiO
2
0.81
La
0.177
Tb
0.003
TiO
2
0.032
Ce
0.499
Dy
0.039
Al
2
O
3
34.75
Pr
0.048
Ho
0.007
Fe
2
O
3
0.4
Nd
0.164
Er
0.021
MgO
0.83
Sm
0.036
Tm
0.007
CaO
0.66
Eu
0.003
Yb
0.037
Na
2
O
9.81
Gd
0.028
Lu
0.007
H
2
O
48
∑REE
1.07
5 CONCLUSIONS
Our investigation indicates that Sinegorsky CO
2
-
rich mineral waters have some unusual geochemical
characteristics including different Ce anomaly. The
NASC-normalized patterns of all groundwaters are
characterized by HREE enrichments and positive Eu
anomalies, whereas some fluids are characterized by
positive Ce anomaly and others have moderate
negative Ce anomaly. All the data are interpreted as
the result of the following processes: 1. REE
leaching in a reducing environment in presence of
CO
2
which enhances concentration of heavy REE; 2.
oxidation of Ce
3+
to Ce
4+
in an oxidizing
environment; 3. deposition and accumulation of
REE (such oxides have positive Ce anomalies
indicating their adsorption of the insoluble Ce-
phase).
The water pumping from boreholes, CO
2
outgassing, changes in the redox conditions and
mineral precipitates deposition are the main
processes thought to control dissolved trace element
behavior by co-precipitation and/or adsorption. It is
suggested that bicarbonate ion-pairs can also play an
important role for the solution chemistry of HREE
which explains the significant relative fractionation
between REE observed in CO
2
-rich water.
Geochemistry of Rare Earth Elements in Sinegorsky Spa of CO2-rich Mineral Water (Sakhalin Island, Far Eastern Russia)
271
ACKNOWLEDGEMENTS
This work was supported by grants from Russian
Science Foundation (RSF), project № 18-17-00245.
REFERENCES
Aubert D, Stille P, Probst A 2001 REE fractionation
during granite weathering and removal by waters and
suspended loads: Sr and Nd isotopic evidence.
Geochim Cosmochim Acta 65 387–406
Bau M 1999 Scavenging of Dissolved Yttrium and Rare
Earths by Precipitating Iron Oxyhydroxide:
Experimental Evidence for Ce Oxidation, Y-Ho
Fractionation and Lanthanide Tetrad Effect Geochim.
Cosmochim. Acta, 63(1) 67–77
Bragin I V, Chelnokov G A, Chudaev O V, Kharitonova
N A, Vysotsky S V 2016 Geochemistry of thermal
waters of continental margin of Far East of Russia
Acta Geologica Sinica 90(1) 276–284
Chelnokov G, Zharkov R, Bragin I 2015 Radon
monitoring in groundwater and soil of Sakhalin island
Journal of geoscience and environment protection 3
48-53 http://dx.doi.org/10.4236/gep.2015.35006
Chelnokov G.A., Bragin I.V., Kharitonova N.A., 2018.
Geochemistry of mineral waters and associated gases
of the Sakhalin Island (Far East of Russia) //Journal of
Hydrology, 559, pp.942-953.
10.1016/j.jhydrol.2018.02.049
Chudaev O V, Bragin I V, Kharitonova N A, Chelnokov
G A 2016 Distribution and Geochemistry of Rare-
Earth Elements in Rivers of Southern and Eastern
Primorye (Far East of Russia) IOP Conference series:
Earth and environmental science 33 (1)
De Baar, H J W, German C R., Elderfield H and van
Gaans P 1988 Rare Earth Element Distributions in
Anoxic Waters of the Cariaco Trench Geochim.
Cosmochim. Acta 52 1203–1219
Gromet L P, Dymek R F, Haskin L A, and Korotev R L
1984 The “North American Shale Composite”: Its
Compilation, Major and Trace Element Characteristics
Geochim. Cosmochim. Acta 12 2469–2482
Johannesson K, Stetchenbach K, Hodge V 1997 Rare
earth elements as ge-ochemical tracers of regional
Geochim. et. Cosmochim. Acta. 61 3605 – 3618
Kharitonova N A, Chelnokov G A, Karabtsov A A and
Kiselev V I 2007 Geochemistry of Na–HCO
3
groundwater and sedimentary bedrocks from the
central part of the Sikhote-Alin mountain region (Far
East of Russia) Applied Geochemistry 22(8) August,
1764-1776
Kharitonova N A, Vakh E A, Chelnokov G A, Chudaev O
V, Aleksandrov I A, Bragin I V 2016 REE
geochemistry in groundwater of the Sikhote Alin fold
region (Russian Far East) Russian Journal of Pacific
Geology 10(2) 141–154
Lewis A J, Palmer M R, Sturchio N C and Kemp A J
1997 The Rare Earth Element Geochemistry of Acid-
Sulfate and Acid-Sulfate-Chloride Geothermal System
from Yellowstone National Park, Wyoming, USA
Geochem. Cosmochim. Acta 61(4) 695–706
Niida K and Kito N 1986 Cretaceous arc-trench systems
in Hokkaido.- in the Monogr. Geology and Tectonics
of Hokkaido.- Sapporo: Assoc. Geol. Collab. Japan,.
31 379-402
Seto M and Akagi A 2008 Chemical condition for the
appearance of a negative Ce anomaly in stream waters
and groundwaters Geochem. J. 42 371–380
Sholkovitz E R 1995 The aquatic chemistry of rare earth
elements in rivers and estuaries Aquat. Geochem. 1
1–34. doi 10.1007/BF01025229
IWEG 2018 - International Workshop on Environment and Geoscience
272
