
Computer Simulations to Predict the Adsorption and Cation
Exchange at Mineral Surfaces
Sen Yang, Qian Wang, Chang Zhu and Gang Yang*
College of Resources and Environment & Chongqing Key Laboratory of Soil Multi-scale Interfacial Process, Southwest
University, Chongqing 400715, China.
Email: theobiochem@gmail.com.
Keywords: Density functional theory, adsorption, clay surface, ion exchange, Hofmeister effects
Abstract: Computer simulations play a critical role in geochemical science. In this work, density functional theory
calculations were employed to address some critical issues that are the adsorption of metal ions and cation
exchange at clay surfaces. Data showed that the adsorption strengths of metal ions at clay surfaces were
enhanced pronouncedly due to the increase of surface charges, while for specific clay particles (with
determined surface charges), the adsorption strengths greatly declined with more loading of metal ions.
Metal ions adsorbed at clay surfaces can be inter-exchanged, and the exchange difficulty significantly relied
on the identity of metal ions and the type of clay minerals. The Hofmeister effects abided by the sequences
of Cs
+
> K
+
> Na
+
> Li
+
on montmorillonite, and kaolinite whereas of Na
+
> Li
+
> Cs
+
≥ K
+
on beidellite.
Based on the cation exchange studies, mechanism of cation exchange at clay surfaces has been proposed.
The cation exchange equilibrium constants were calculated for the various alkali ions at the surfaces of
different clay minerals, which are in accord with the experimental observations.
1 INTRODUCTION
Adsorption of metal ions at the interface of clay
minerals and aqueous solutions largely controls the
distribution, transport, and bioavailability of
nutrients, water, and contaminants. It has been
acknowledged as a critical topic for a number of
disciplines such as chemical, environmental and
geological sciences (Sposito et al., 1999; Dahn et al.,
2003). Cation exchange is closely associated with
adsorption. Heavy (e.g., Pb
2+
) and radioactive (e.g.,
Pb
2+
) metal ions adsorbed at clay surfaces can be
exchanged with regular metal ions (e.g., K
+
), which
further remediates the polluted geological resources
(Loganathan and Kalinichev, 2017).
Computer simulations have gained a great deal
of valuable information such as adsorption
configurations, microscopic interactions, dynamics,
and diffusion that are otherwise inaccessible. Zhang
et al. (Zhang et al., 2017) used first-principles
molecular dynamics simulations and found that Ni
2+
forms three different complexes with the edge
structures of montmorillonite. They were
respectively the monodentate binding at the ≡SiO
site, bidentate binding at the ≡Al(OH)
2
site and
tetradentate binding at the octahedral vacancy with
Ni
2+
fitting well into the clay lattice. The tetradentate
complex is significantly preferred and can be
deprotonated at normal conditions (pKa = 8.4) while
the other less stable complexes are obviously more
difficult to be deprotonated owing to the extremely
high pKa values. Wang et al. (Wang et al., 2017)
demonstrated that six factors affected the adsorption
of K
+
at clay surfaces. The quantity of negative
charges is the most critical to decide the adsorption
performances while the other factors under certain
circumstances can also play an important role. Clay
minerals generally carry an abundance of negative
charges that result in strong surface electric fields
(Kumar et al., 2016; Calarge et al., 2013). In
presence of strong electric fields, metal ions are
inner-sphere adsorbed at the interface of clay
minerals and aqueous solution, which resemble
closely the adsorption configurations under dry
conditions (Zhang et al., 2017; Wang et al., 2017;
Tian et al., 2015).
The previous studies (Wang et al., 2017; Li et al.,
2017) showed that density functional theory
314
Yang, S., Wang, Q., Zhu, C. and Yang, G.
Computer Simulations to Predict the Adsorption and Cation Exchange at Mineral Surfaces.
In Proceedings of the International Workshop on Environment and Geoscience (IWEG 2018), pages 314-318
ISBN: 978-989-758-342-1
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
calculations were presently employed to study the
adsorption of K
+
ions at montmorillonite surfaces. In
addition to the adsorption of a single K
+
ion at clay
minerals with different negative charges as reported
previously (Wang et al., 2017; Li et al., 2017),
disparate numbers of K
+
ions (n = 13) were
considered to inspect the correlation between the
adsorption performances vs. the number of metal
ions. As observed experimentally, more than one
metal ion should be bound onto clay surfaces at the
same time. A portion of these adsorbed metal ions
can be exchanged and the exchange degree should
depend on the identities of two metal ions involved
in the exchange processes. The second issue of this
work was to address the exchange thermodynamics
among the various alkali ions on three clay surfaces
(montmorillonite, kaolinite, and beidellite). Then the
mechanism of cation exchange at clay surfaces was
tentatively proposed. Finally, the cation exchange
equilibrium constants for the various alkali ions at
clay surfaces were calculated and compare with the
experimental results.
2 COMPUTATIONAL SECTION
First-principles density functional theory
calculations were performed using Gaussian09
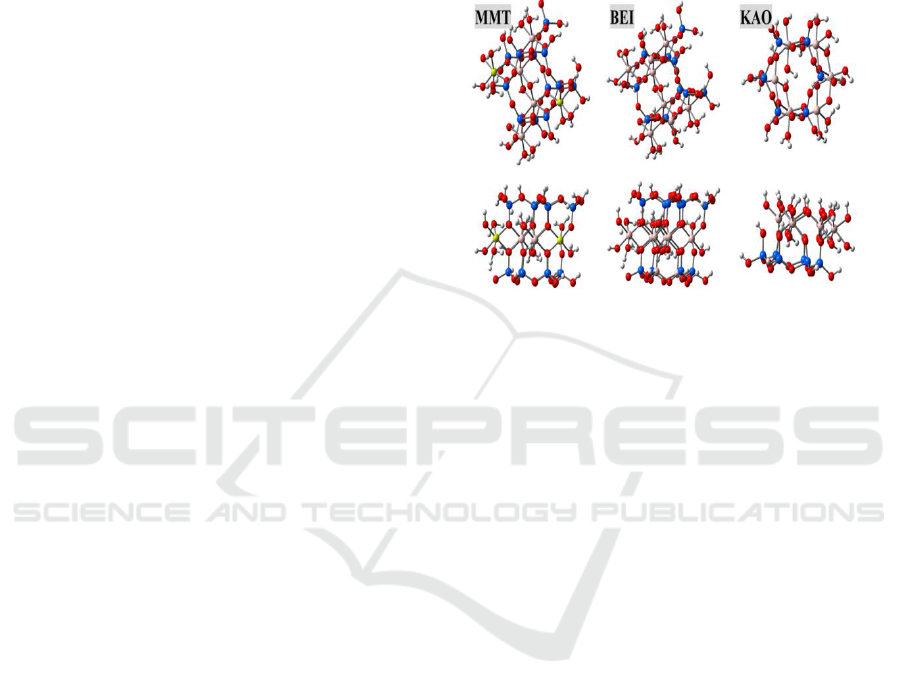
software packages (Frisch et al., 2013). Figure 1
showed the models for montmorillonite, kaolinite,
and beidellite. Almost all clay minerals were
characterized by an abundance of surface charges
(Kumar et al., 2016; Calarge et al., 2013). Clay
minerals presently used were endowed with -2
charges, unless otherwise noted. As suggested
(Wang et al., 2017), metal ions were adsorbed on the
external surfaces of clay minerals. In line with
previous literatures (Tian et al., 2015; Jia et al.,
2018), the hexagonal rings of silica surfaces along
with adsorbed metal ions were selected as the high-
level region that was handled at the B3LYP/6-
31+G(d,p) level of theory, while the rest of models
were treated as the low-level region and simulated
by the B3LYP/3-21G method. Owing to the
significance of relativistic effects, the inner and
valence electrons of Cs were described by the
LanL2DZ effective core potential and LanL2DZ
basis set, respectively.
3 RESULTS AND DISCUSSION
3.1 K
+
adsorption onto
Montmorillonite Surfaces
Top (upper panel) and side (lower panel) views of
models are shown, and Si, Al, Mg, O and H are
presented in blue, pink, yellow, red and white balls,
respectively.
Figure 1: Models of montmorillonite (MMT), beidellite
(BEI) and kaolinite (KAO).
With the increase of negative charges in
montmorillonite caused by more Mg
2+
/Al
3+
substitutions, the distances of K
+
to the adjacent
surface-O atoms (O
S
) showed a gradual reduction,
consistent with the previous results (Wang et al.,
2017; Tian et al., 2015). For (the number of
negative charges) = 1, 2, 3, the distances of K
+
to
three closet O
S
atoms were averaged at 2.734, 2.684
and 2.657 Å, suggesting that there were stronger
interactions between metal ions and clay surfaces
due to the increase of negative charges. The
interaction energies between K
+
and the
montmorillonite models were calculated to be -522.5,
-739.6 and -976.8 kJ/mol, respectively, and the
adsorption performances of metal ions were
substantially enhanced at clay surfaces with more
negative charges (Wang et al., 2017; Tian et al.,
2015). It demonstrated that the quantity of negative
charges plays a critical role during the adsorption of
metal ions (Wang et al., 2017; Li et al., 2017).
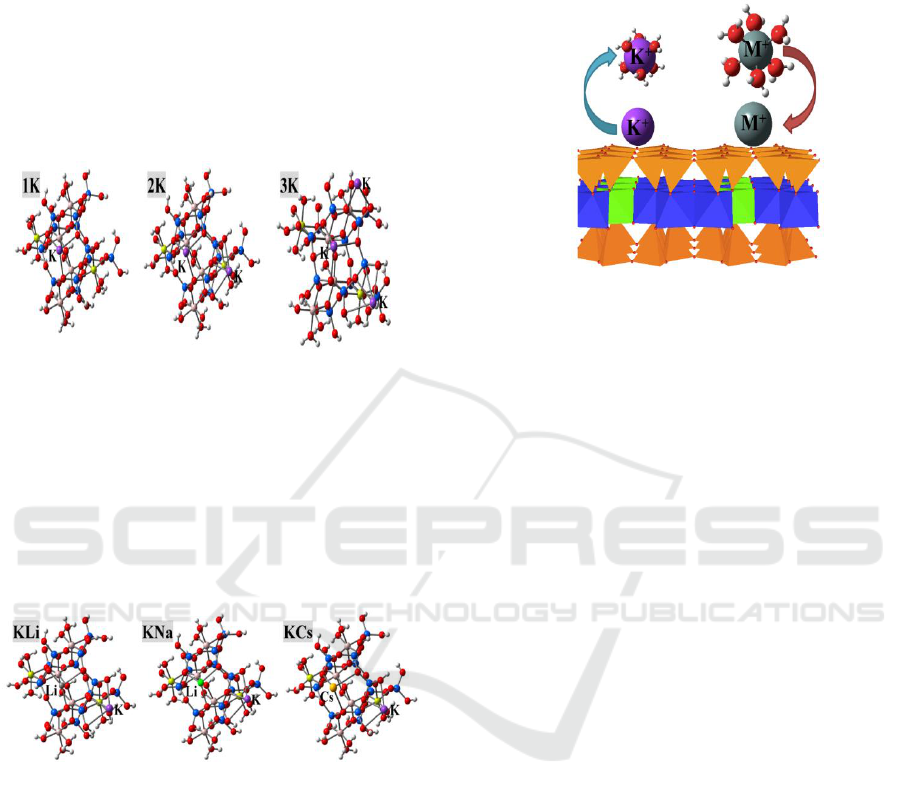
Figure 2 depicted the adsorption configurations
of one, two and three K
+
ions onto the surfaces of
montmorillonite with two Mg
2+
/Al
3+
substitutions
(Calarge et al., 2013). Compared with the first K
+
ion (n = 1), the adsorption strengths of subsequent
K
+
ions considerably reduced, and the interactions
Computer Simulations to Predict the Adsorption and Cation Exchange at Mineral Surfaces
315
energies between the first, second and third K
+
ions
and the montmorillonite surfaces amounted to -
739.6, -379.6 and -131.4 kJ/mol, respectively. It
clearly indicates that for given clay particles, the
latter adsorbed metal ions are more facile to be
detached from their surfaces and as the desorption
process continues, metal ions are getting more and
more difficult to desorb.
Figure 2: Structures for the adsorption of one, two and
three K
+
ions at montmorillonite surfaces.
Si, Al, Mg, O, K and H are presented in blue, pink,
yellow, red, purple and white balls, respectively.
3.2 Cation Exchange Thermodynamics
and Mechanism
Figure 3: Structures for the adsorption of Li
+
, Na
+
and
Cs
+
at K
+
- montmorillonite surfaces
Si, Al, Mg, O, K, Li, Na, Cs and H are presented in blue,
pink, yellow, red, purple, grey, green, orange and white
balls, respectively.
Figure 3 showed the structures of 2K (Figure 2)
exchanged by one other alkali ion M
+
= Li
+
, Na
+
,
Cs
+
). The adsorption configurations of different
alkali ions at clay surfaces resembled closely each
other. Two different mechanisms for cation
exchange were given,
2K + M
+
(bare) → KM + K
+
(bare) (1)
2K + M
+
(aq.) → KM + K
+
(aq.) (2)
where bare and aquation in parentheses manifest the
bare and aqueous conditions, respectively.
Scheme 1: Mechanism posed for cation exchange
(M+/K+) at clay surfaces.
The cation exchange energies were calculated
and are presented in Table 1. When the bare metal
ions were used (eq. 1), the cation exchange energies
were -176.4, -96.4 and 81.7 kJ/mol for Li
+
, Na
+
and
Cs
+
, respectively. The Hofmeister sequence based
on the bare metal ions was presented as Li
+
> Na
+
>
K
+
> Cs
+
, apparently differing from the experimental
observations (Tian et al., 2015). When the aqueous
species were used (eq. 2), the cation exchange
energies amounted to 14.3, 5.1 and -13.3 kJ/mol for
Li
+
, Na
+
and Cs
+
, respectively, where a consistent
Hofmeister series with the experimental
observations (Cs
+
> K
+
> Na
+
> Li
+
) was attained.
Consequently, the mechanism for cation exchange at
clay surfaces should comply with eq. 2 and was
further sketched in Scheme 1, which got support
from the recent molecular dynamics simulations
(Loganathan and Kalinichev, 2017) and the
following discussions.
The cation exchange energies (M
+
/K
+
) at the
surfaces of beidellite and kaolinite were also
calculated (Table 1). The Hofmeister sequences
followed as Na
+
> Li
+
> Cs
+
≥ K
+
and Cs
+
> K
+
>
Na
+
> Li
+
for beidellite and kaolinite, respectively.
Accordingly, disparate clay minerals may
correspond to distinct Hofmeister series and cation
exchange phenomena. The Hofmeister sequences
during ion exchange and clay aggregation were
known to be affected by a number of factors (Tian et
al., 2015;Li et al., 2017; Jia et al., 2018), and the
experimental verifications on these calculated results
are anticipated in the near future.
IWEG 2018 - International Workshop on Environment and Geoscience
316
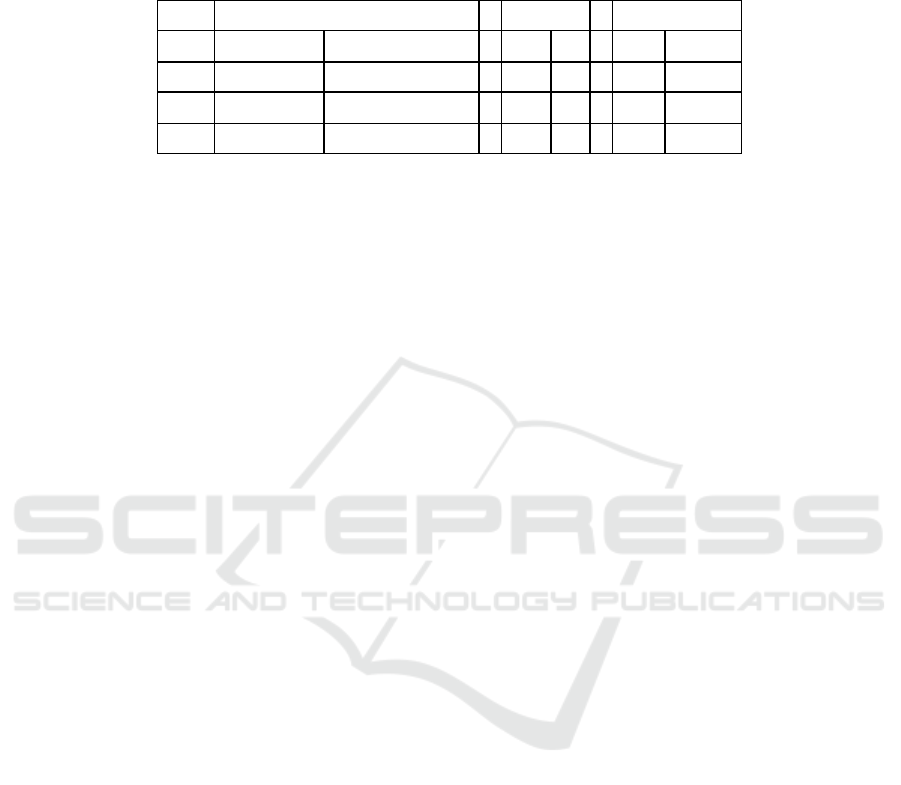
Table 1: Cation exchange energies (∆Eex) and cation exchange equilibrium constants (K
ex
) for different K
+
-clays
a, b
.
montmorillonite
beidellite
kaolinite
∆E
ex
K
ex
∆E
ex
K
ex
∆E
ex
K
ex
Li
+
14.3 (-176.4)
0.0031 (8.0 x 10
30
)
-1.8
2.0
17.8
0.00076
Na
+
5.1 (-96.4)
0.13 (7.8 x 10
16
)
-3.5
4.1
7.5
0.048
Cs
+
-13.3 (81.7)
217.8 (4.9 x 10
-15
)
-0.8
1.4
-13.6
244.9
a
Energy units in kJ/mol;
b
Data using bare metal ions are shown in parentheses.
3.3 Cation Exchange Equilibrium
Constants
It is difficult for experimentalists to refer to and
make comparisons with the calculated cation
exchange energies. However, the cation exchange
equilibrium constants (K
ex
) can provide a good
platform to bridge the computational and
experimental results. The cation exchange
equilibrium constants (K
ex
) and cation exchange free
energies (∆G
ex
) can be correlated by the Arrhenius
equation [3], i.e.,
∆G
ex
= -RTlnK
ex
(3)
where R and T stand for gas constant and
temperature, respectively.
We know that ∆G
ex
= ∆H
ex
− T∆S
ex
= ∆E
ex
+ p∆V
ex
− T∆S
ex
(4)
where H, S, p, and V are enthalpy, entropy,
pressure, and volume, respectively.
For cation exchange at clay surfaces, the
volumes of clays remained essentially invariable
(∆V
ex
0), and because the clay structures prior and
posterior to cation exchange (Figures 2 and 3)
closely resembled each other, the entropic effects
can almost be neglected (∆S
ex
0). Accordingly, the
cation exchange energies (∆E
ex
) were close to the
cation exchange free energies (∆G
ex
) and were used
for calculating the cation exchange equilibrium
constants (K
ex
). The cation exchange equilibrium
constants (K
ex
) (Table 1) can be compared directly
with the experimental results: Hanshaw (Hanshaw,
1963) and Crooks et al. (Crooks et al., 1993)
determined that K
ex
approximates 0.2 and 0.060.12
for Na
+
/K
+
, respectively, for montmorillonite, which
is in good agreement with the present value of 0.13
and further demonstrated the cation exchange
mechanism posed in Scheme 1. Table 1 showed the
Na
+
/K
+
exchange equilibrium constant using the
bare metal ions was calculated to be 7.8 x 10
16
and
deviated remarkably from the experimental data.
4 CONCLUDING REMARKS
We used density functional theory calculations to
address some critical issues that are the adsorption
of metal ions and cation exchange at clay surfaces.
The interaction energies of metal ions at the clay
surfaces were pronouncedly enhanced due to the
increase of surface charges suggesting the reinforced
adsorption strengths, while for given clay particles
(with determined surface charges), the adsorption
strengths declined greatly with the increased loading
of metal ions. Accordingly, the latter adsorbed metal
ions were more facile to be detached from clay
surfaces.
Metal ions adsorbed at clay surfaces can be inter-
exchanged, and the exchange difficulty significantly
relied on the identity of metal ions and the type of
clay minerals. The Hofmeister effects abided by the
sequence of Cs
+
> K
+
> Na
+
> Li
+
for
montmorillonite that agreed finely with the
experimental observations. The Hofmeister
sequence remained for kaolinite while was altered
substantially for beidellite where the sequence was
presented as Na
+
> Li
+
> Cs
+
≥ K
+
.
The mechanism of cation exchange at clay
surfaces has been posed and got strong support from
the present density functional theory and previous
molecular dynamics results. The cation exchange
equilibrium constants were calculated for the various
alkali ions at the surfaces of montmorillonite,
beidellite, and kaolinite that are in line with the
experimental observations available.
Computer Simulations to Predict the Adsorption and Cation Exchange at Mineral Surfaces
317

ACKNOWLEDGEMENTS
This work was sponsored by the National Natural
Science Foundation of China (21473137 and
41530855), the Fourth Excellent Talents Program of
Higher Education in Chongqing (2014-03) and the
Natural Science Foundation Project of CQ CSTC,
China (cstc2017jcyjAX0145).
REFERENCES
Calarge L M, Meunier A and Formoso M L L 2013 A
bentonite bed in the Aceguá (RS, Brazil) and Melo
(Uruguay) areas: A highly crystallized montmorillonite.
J. South Am. Earth Sci. 16 187
Crooks J E, El-Daly H, El-Sheikh M Y, Habib A M and
Zaki A B 1993 Kinetics of ion ‐ exchange on
montmorillonite clays. Int. J. Chem. Kinet. 25 161
Dahn R, Scheidegger A M, Manceau A, Schlegel M L,
Baeyens B, Bradbury M H and Chateigner D 2003
Structural evidence for the sorption of Ni(II) atoms on
the edges of montmorillonite clay minerals: A
polarized X-ray absorption fine structure study.
Geochim. Cosmochim. Acta 67 1
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E,
Robb M A and Cheeseman J R, et al. 2013 Gaussian
09, Revision D.01, Gaussian, Inc., Wallingford CT
Hanshaw B B 1963 Cation-exchange constants for clays
from electrochemical measurements. Clays Clay
Minerals 12 397
Jia Z Q, Li X, Zhu C, Yang S and Yang G 2018 Reversal
of cation-specific effects at the interface of mica and
aqueous solutions. J. Phys. Chem. C 122 5358
Kumar N, Zhao C L and Klaassen A van den Ende D,
Mugele F and Siretanu I 2016 Characterization of the
surface charge distribution on kaolinite particles using
high resolution atomic force microscopy. Geochim.
Cosmochim. Acta 175 100
Li X, Li H and Yang G 2017 Electric fields within clay
materials: How to affect the adsorption of metal ions. J.
Colloid Interf. Sci. 501 54
Loganathan N and Kalinichev A G 2017 Quantifying the
mechanisms of site-specific ion exchange at an
inhomogeneously charged surface: Case of Cs
+
/K
+
on
hydrated muscovite mica. J. Phys. Chem. C 121 7829
Sposito G, Skipper N T, Sutton R, Park S H, Soper A K
and Greathouse J A 1999 Surface geochemistry of the
clay minerals. Proc. Natl. Acad. Sci. USA 96 3358
Tian R, Yang G, Tang Y, Liu X M, Li R, Zhu H L and Li
H 2015 Origin of Hofmeister effects for complex
systems. PLoS One 10 e0128602
Wang Q, Zhu C, Yun J N and Yang G 2017 Isomorphic
substitutions in clay materials and adsorption of metal
ions onto external surfaces: A DFT investigation. J.
Phys. Chem. C 121 26722
Zhang C, Liu X D, Lu X C, He M J, Meijer E J and Wang
R C 2017 Surface complexation of heavy metal cations
on clay edges: Insights from first principles molecular
dynamics simulation of Ni(II). Geochim. Cosmochim.
Acta 203 54
IWEG 2018 - International Workshop on Environment and Geoscience
318
