
Preparation of Co-conjugate Microporous Polymer Magnetic
Tubular Composites and Application in Removal of
Phosphate Ions
S Li, Z L Hu, Z Q Zhu, H X Sun and A Li
*
College of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou,
China
Corresponding author and e-mail: A Li, lian2010@lut.cn
Abstract. On the basis of synthesizing tubular conjugated microporous polymer, Co
nanoparticles were synthesized in situ on the surface and inside of the tube for the removal of
phosphate ions in water. Under the conditions of 24 hours of adsorption, the removal rate of
an aqueous solution containing 100 mg L
-1
of phosphate ion can reach 82.77%, and the
requirement for separating contaminants can be achieved by applying a magnetic field. It is
proved that the composite material has a good effect of purifying phosphorus-contaminated
water sources.
1. Introduction
Since the beginning of the 21st century, with the advancement of science and technology, the
pollution of water by many organic pollutants has gradually increased, such as the presence of
phosphorus in pesticides, the pollution of aromatic raw materials in industrial production, and so on.
Due to the richness of phosphorus, the enrichment of water bodies and lakes is still called an
important environmental issue [1-4]. The reduction of phosphorus emissions and the reduction of the
impact of these pollutions on water have also become a hot research direction for scientists today.
People have proposed numerous solutions to the above problems. Among the numerous solutions,
the adsorption method is widely used due to its unique mechanism [5-6]. In addition, the adsorption
method has many advantages such as high efficiency, safety, economy, and simple operation. Finding
stable and efficient adsorbents becomes the key to the application of adsorption methods. The
characteristics of the adsorption method determine that the adsorbent should have a high specific
surface area, excellent stability, and can be recycled and used. The porous material has a large
specific surface area, and its unique pore structure makes it widely used in the field of adsorption
[7-10].
Organic porous materials have a series of advantages. Due to its unique pore structure, high
specific surface area, π-π-conjugated chemical structure, ultra-high thermal and chemical stability,
and simple synthesis methods, conjugated microporous polymers are A series of adsorption
experiments have been greatly developed [11-13]. In 2015, our project was combined into a unique
tubular and porous structure of conjugated microporous polymer [14]. Based on this, this chapter
uses tubular conjugated microporous polymer as the carrier to load Co nanoparticles into it. In the
302
Li, S., Hu, Z., Zhu, Z., Sun, H. and Li, A.
Preparation of Co-conjugate Microporous Polymer Magnetic Tubular Composites and Application in Removal of Phosphate Ions.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 302-306
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

adsorption of phosphate ions has been greatly improved, and can be achieved through the external
magnetic field, the effect of rapid separation.
2. Experimental section
2.1. Materials
Solid reagents are 1,3,5-triethynylbenzene, 2-amino-3,5-dibromopyridine, tetrakis
(triphenylphosphine) palladium (0), copper (I) iodide, cobalt trichloride hexahydrate, sodium
hydroxide. Liquid reagents are toluene, trimethylamine, chloroform, acetone, methanol, hydrated
hydrazine, ethanol and all organic solvents were used as received.
2.2. Methods
(1) 337.86 mg 1,3,5-triethynylbenzene, 750.9 mg 2-amino-3,5-dibromopyridine, 150 mg tetrakis
(triphenylphosphine) palladium(0), 50 mg copper(I) iodide reacts under nitrogen protection to form
tubular-shape conjugated microporous polymers. named CMP. Then the mixture was washed with
chloroform, water, acetone, and methanol for several times and named CMP. (2) The magnetic
composites were synthesized in 20mL ethanol and 5% sodium hydroxide solution by 0.1g CMP and
0.8g cobalt trichloride hexahydrate. At last, the samples were centrifuged, washed, dried and named
Co-CMP.
2.3. Removal of phosphate ions
According to the Lambert-Beer law to configure the aqueous solutions of different concentrations of
phosphate ions. Weigh a certain amount of Co-CMP (10 mg) into a glass vial, add 20 mL of an
aqueous solution of 100 mg L-1, 300 mg L-1 and 500 mg L-1 phosphate ion, and seal the glass vial
and place it in a shaker for 24 h. Adsorption reaches equilibrium.
Under strong acidic conditions, the active phosphate in the water sample reacts with ammonium
molybdate to produce pale yellow phosphomolybdate. Phosphomolybdate yellow is reduced by
stannous chloride (SnCl) to blue phosphorous molybdenum blue. The blue depth is proportional to
the active phosphate content and has a maximum absorption at 710 nm. The colorimetric method can
be used to measure the content of active phosphate in the water sample.
After that the absorbance of the supernatant liquids was determined by UV-Vis spectrophotometer,
which was described as:
Eq.1: Removal rate= (A1-A2)/A1·100%
A1: the initial absorbency of phosphate ions, A2: the absorbency of phosphate ions within time
24h.
3. Results and discussion
3.1. Analysis of the composites appearance and TEM
As can be seen from the (a) transmission electron microscope, the CMP has a hollow tubular
structure and has good dispersibility. From the TEM image of Co-CMP in Figure 1 (b), it can be
further seen that Co particles grow not only on the surface of the loaded surface CMP but also in the
interior of the tube, indicating that Co particles are in the process of in-situ synthesis. Tube surface
load is successful.
Preparation of Co-conjugate Microporous Polymer Magnetic Tubular Composites and Application in Removal of Phosphate Ions
303
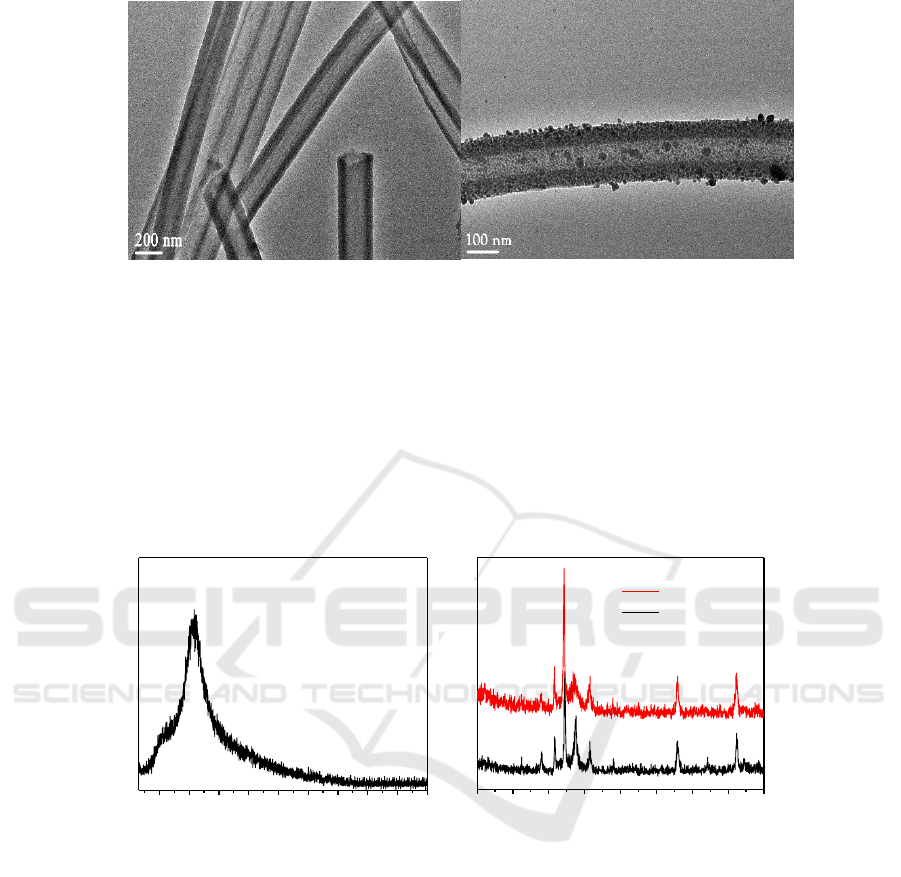
Figure 1. (a) CMP transmission electron microscopy picture, (b) Co-CMP transmission electron
microscopy picture.
3.2. Analysis of the composites XRD
The CMP XRD pattern has a large broad peak near 22o, a broad peak of a typical amorphous
polymer, and is amorphous. The X-ray diffraction curves of Co and Co-CMP composites are shown
in Figure 2 (b), 2θ=41.5o (100), 44.1o (002), 47.4o (102), 51.22o (200), 75.7o (220), 92.2o (311) are
characteristic peaks of Co particles [15]. The XRD pattern of Co-CMP was compared with the XRD
pattern of Co. Irregular peaks appeared at 20-30o due to the presence of CMP tubes, indicating the
synthesis of Co-CMP composites.
10 20 30 40 50 60 70 80 90 100
Intensity
2 Theta (degree)
(a)
20 30 40 50 60 70 80 90 100
Co-CMP
Co
Intensity
2 Theta (degree)
(b)
Figure 2. (a) X-Ray Diffraction Curves of CMP, (b) X-Ray Diffraction Curves of Co (Black) and
Co-CMP (Red).
3.3. Analysis of the composites materials TGA
The thermal stability analysis of CMP, Co, and Co-CMP is shown in Figure 3 (a). Under the nitrogen
protection conditions, the final mass loss of Co particles is about 3 wt%, which is the residual organic
matter on the surface of the particles. In CMP, there is basically no mass loss before 300°C. After
300°C, the mass eventually decreases to about 75.5 wt% with increasing temperature. The reason for
its good stability is that the CMP structure is composed of a rigid aromatic ring coupled with a
relatively strong covalent bond. The figure shows that the final remaining mass of Co-CMP is 91.5
wt%. By the ratio calculation, the CMP content is about 22.4 wt%, and the Co particle content is 75
wt%. CMP provides a good skeletal structure and pore structure, with Co particles loaded on its
surface.
3.4. Analysis of the composites materials VSM
Figure 3 (b) shows the hysteresis regression curves of Co and Co-CMP composites at room
(a)
(b)
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
304
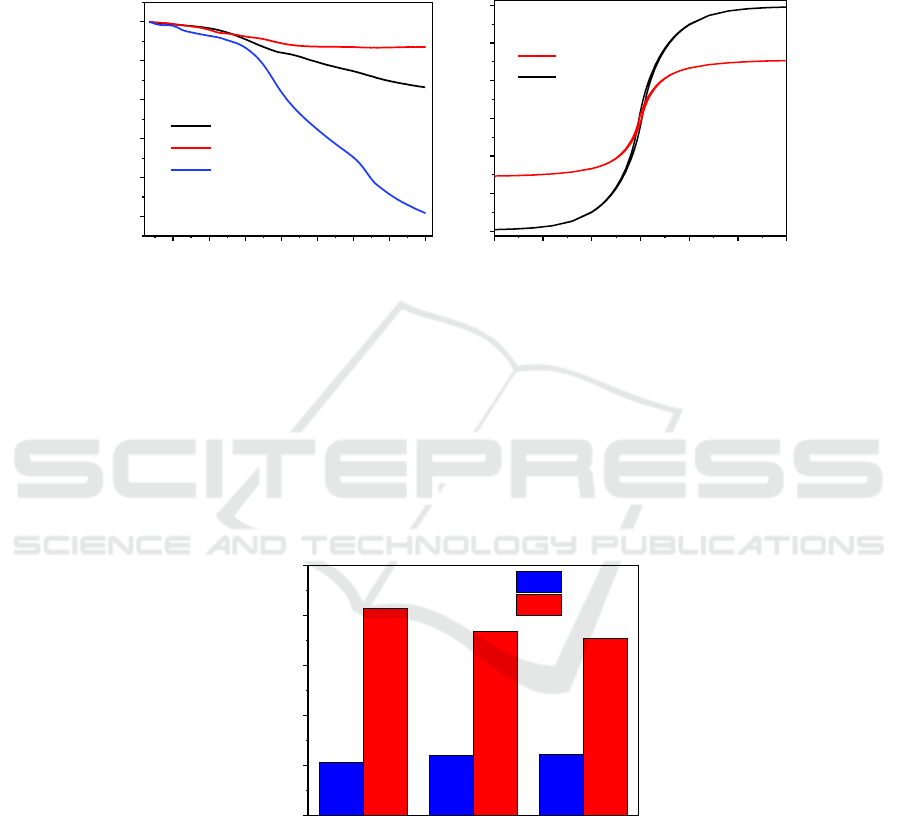
temperature (298 K). It shows that both Co and Co-CMP composites have good saturation magnetic
field strength at room temperature. The magnetization was 118.1 and 61.3 emu g-1, respectively. Due
to the presence of tubular CMP, the resulting polymer is reduced in magnetic properties, but at the
same time it maintains good magnetic properties. With the change of CMP quality, the magnetic
strength of Co-CMP composites can be controlled to ensure its separation effect.
100 200 300 400 500 600 700 800
75
80
85
90
95
100
Co-CMP
Co-2
CMP
Relative weight (%)
Tempurature (
o
C)
(a)
-15000 -10000 -5000 0 5000 10000 15000
-120
-80
-40
0
40
80
120
Magnetic Field (Oe)
Co-CMP
Co
Magnetization emu
-1
(b)
Figure 3. (a) Thermogravimetric analysis of Co-CMP (black), Co-2 (red), CMP (blue) (b) Hysteresis
curve of Co (black) and Co-CMP (red).
3.5. UV-vis results for phosphate ion removal
In the 24-hour adsorption experiment, the removal rates of the aqueous solutions containing 100 mg
L-1, 300 mg L-1, and 500 mg L-1 of phosphate ions were 21.4%, 24.1%, and 24.64%, respectively.
Figure 4 shows the removal rate of Co-CMP was 82.77%, 73.8% and 70.72%. It can be seen that
there is a significant increase in the ability of the removal of phosphate ions in Co-CMP compared to
CMP.
100 300 500
0
20
40
60
80
100
Co
Co-CMP
82.77
70.72
73.8
24.64
24.1
Removal rate (%)
Phosphate ion content (mg L
-1
)
21.4
Figure 4. Removal rate of phosphate ion in an aqueous solution by CMP and Co-CMP.
4. Conclusions
A magnetic tubular composite material, Co-CMP, was synthesized and it can effectively adsorb
phosphate ions. Phosphorus-polluted water bodies are purified and the contaminants are separated by
an external magnetic field. In addition, when the aqueous solution with a phosphate ion content of
100 mg L-1 is treated for 24 hours, the removal rate can reach 82.77%.
Preparation of Co-conjugate Microporous Polymer Magnetic Tubular Composites and Application in Removal of Phosphate Ions
305

Acknowledgement
The authors are grateful to the National Natural Science Foundation of China (Grant No. 51663012,
51462021), Project of Collaborative Innovation Team, Gansu Province, China and Innovation and
Entrepreneurship Talent Project of Lanzhou (2017-RC-33).
References
[1] Jin X, Wang S, Pang Y, Zhao H and Zhou X 2005 Colloids Surfaces A Phy. Eng.
Aspects 254(1–3) 241-248
[2] Galarneau E and Gehr R 1997 Water Res. 31(2) 328-338
[3] Zhou Q, Wang X, Liu J and Zhang L 2012 Chem Eng. J. 200-202(16) 619-626
[4] Ashekuzzaman S M and Jiang J Q 2014 Chem Eng. J. 246(12) 97-105
[5] Cheng X, Huang X, Wang X and Sun D 2010 Ciesc J. 61(4) 955-962
[6] Das J, Patra B S, Baliarsingh N and Parida K M 2006 Appl Clay Sci. 32(3-4) 252-260
[7] Wang F, Ren F, Mu P, Zhu Z Q, Sun H X and Ma C H 2017 J Mater Chem A 5(22)
11348-11356
[8] Qian X, Zhu Z Q, Sun H X, Ren F, Mu P and Liang W D 2016 ACS Appl. Mater Inter 8(32)
21063-21069
[9] Qian X, Wang B, Zhu Z Q, Sun H X, Ren F and Mu P 2017 J. Hazard Mater 338 224-232
[10] Ren F, Zhu Z Q, Qian X, Liang W D, Mu P and Sun H X 2016 Chem Commun 52(63)
9797-9800
[11] Cooper A I 2009 Adv. Mater 21(12) 1291-1295
[12] Ren S, Bojdys M J, Dawson R, Laybourn A, Khimyak Y Z and Adams D J 2012 Adv.
Mater 24(17) 2357-2361
[13] Zhang Q, Sun H X, Wang X, Zhu Z Q, Liang W D and Li A 2013 Energy Technol-gel 1(12)
721-725
[14] Bao L L, Sun H X, Zhu Z Q, Liang W D, Mu P and Zang J K 2016 Mater Lett 178 5-9
[15] Sun S and Murray C B 1999 J. Appl. Phys. 85 4325-4330
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
306
