
Synthesis of Single Crystal ZSM-5 by Solid-like State
Conversion
S Chen, S Y Han, Y Liu, D D Guan, N Z Jiang and W Meng
*
Department of Chemistry, College of Science, Yanbian University, Yanji, Jilin,
133000, P. R. China.
Corresponding author and e-mail: W Meng, mengw@ybu.edu.cn
Abstract. ZSM-5 single crystals were synthesized from fumed silica via solid-like state
conversion. The properties of synthesized zeolite samples were characterized by XRD, SEM,
27
Al MAS NMR spectra, XRF and NH
3
-TPD. The results showed that the amorphous solid
phase could be converted into ZSM-5 zeolites with cubic-like crystal morphology at
temperature range of 160
o
C-190
o
C within 24 h. The optimum synthesis condition is at 180
o
C
for 16 h by considering the crystallinity and uniformity of zeolites. The conversion of toluene
in gas phase disproportionation over optimized ZSM -5 catalyst raised to 40.18%, which is
higher than commercial ZSM-5 zeolite.
1. Introduction
The ZSM-5 zeolites have been studied for a long time as solid acid catalysts in the petrochemical and
fine chemical industries due to features such as large specific surface area, excellent shape selectivity,
and high hydrothermal stability [1]. However, conventional hydrothermal synthesis of ZSM-5
zeolites involves the use of large amounts of water as solvent, which consumes lots of energy, and
causes water pollution. Therefore, considerable efforts have been dedicated to developing a less
water synthetic approach to produce ZSM-5 zeolite [2-3].
In 1990, Xu et al. [4] firstly reported the synthesis of ZSM-5 via dry-gel conversion (DGC). The
DGC method could obviously reduce the requirement of water, enhance the yield of the products,
and shorten the crystallization time [5-6]. In 2012, Xiao et al. [7] synthesized zeolites in the absence
of any solvent (solvent-free method) by mixing, grinding, and heating solid raw materials. But, it
requires chemical raw agents rich of crystalline water. In 2014, Lu et al. [8] reported a fast route for
synthesizing of nano-sized ZSM-5 aggregates from leached metakaolin via solid-like state
conversion. Before the crystallization, the solid raw materials were mixed in the presence of small
amount of water.
In this work, the crystallization time and temperature of solid-like state conversion were studied to
synthesize uniform ZSM-5 single crystals. The samples were characterized using X-ray powder
diffraction (XRD), scaning electron microscopy (SEM),
27
Al MAS NMR spectra, X-Ray
fluorescence (XRF) spectrometer and temperature programmed desorption (NH
3
-TPD). The zeolites
synthesized showed comparable catalytic performance to commercial zeolite on the toluene
disproportionation.
330
Chen, S., Han, S., Liu, Y., Guan, D., Jiang, N. and Meng, W.
Synthesis of Single Crystal ZSM-5 by Solid-like State Conversion.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 330-335
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2. Experiments
2.1. Material
All the reagents used for the preparation of ZSM-5 zeolite were aluminium sulfate (Al
2
(SO
4
)
3
·18H
2
O,
Tianjin Branch Miou Technology Co., Ltd.), fumed silica (Sigma-Aldrich), tetrapropylammonium
bromide (TPABr, Aladdin) and sodium hydroxide (NaOH, Shenyang Xinhua Reagent Factory
products) as alkali source. Commercial zeolite (ZSM-5, SiO
2
/Al
2
O
3
=45-80) were purchased from
Nanjing Jicang Nano Technology Co., Ltd.
2.2. Preparation of catalysis
The standard molar ratio of the solid raw materials was Al
2
O
3
: SiO
2
: NaOH: TPABr: H
2
O=0.02: 1:
0.60: 0.04: 1.0. In a typical synthesis, 1.68 g fumed silica, 0.38 g Al
2
(SO
4
)
3
·18H
2
O, 0.35 g TPABr,
0.67 g NaOH and 0.50 g H
2
O were mixed by grinding, transferred into a 100 mL Teflon-lined
autoclave, heated at X
o
C (X=160, 170, 180, 190) for Y h (Y=8, 16, 20, 24) and the samples were
designated as S-X-Y. After cooling the autoclave to room temperature, the solid sample was washed
with deionized water until pH=7, then dried overnight at 80
o
C, and calcined at 550
o
C for 6 h to
remove the template. Zeolite sample was ion-exchanged for 3 times in NH
4
Cl solution (1.5 mol/L)
with the solid/solution weight ratio of 0.1 at 90
o
C for 3 h, and then calcined again at 550
o
C for 6 h to
obtain the corresponding H-type ones.
2.3. Characterization
X-ray diffraction (XRD) patterns was performed on a Bruker D8 diffractometer, using Cu K
α
radiation in the 2θ range of 5-50° with an angular step size of 0.02°. The relative crystallinity was
calculated according to the method described by Paris [9]. The morphology of the samples were
characterized by SEM on a scanning electron microscopy (SEM, SU8010, Hitachi).
27
Al magic angle
spinning nuclear magnetic resonance (MAS NMR, AVANCE
Ⅲ
, Bruker) spectroscopy
characterization was performed on a Bruker DSX 500 MHz spectrometer with a spinning rate of 14
kHz and a length of 1 MS. The acid sites were determined by NH
3
temperature-programmed
desorption (NH
3
-TPD) on a Pulse Chemisorb 2705 (Micromeritics, USA) instrument. Chemical
compositions of the samples were determined using an X-Ray fluorescence (XRF) spectrometer
(Epsilon3, Panalytical).
2.4. Catalytic activity test
Catalytic reactions were conducted in a quartz tube reactor of 8 mm ID and 600 mm length under
atmospheric pressure with a weight hourly space velocity (WHSV) of 3.3 h
-1
. The reaction products
(toluene, xylene isomers, etc.) were analysed using a gas chromatograph-mass spectrometer (GC-MS)
equipped with a flame ionization detector (FID) for 6 h
3. Results and discussion
3.1. Factors affecting the synthesis of ZSM-5 zeolites
3.1.1 Effect of crystallization temperature
1) XRD characterization results
Crystallization temperature strongly affects the nucleation and crystal growth [10]. Figure 1 shows
XRD patterns of the samples synthesized at different temperatures for 24 h. The characteristic
diffraction peaks of ZSM-5 (2θ=7-9°, 22-25°) began to appear at 160
o
C, and their intensity gradually
increased while crystallization temperature was raised to 180
o
C, but decreased at further increased
temperature of 190
o
C.
Synthesis of Single Crystal ZSM-5 by Solid-like State Conversion
331
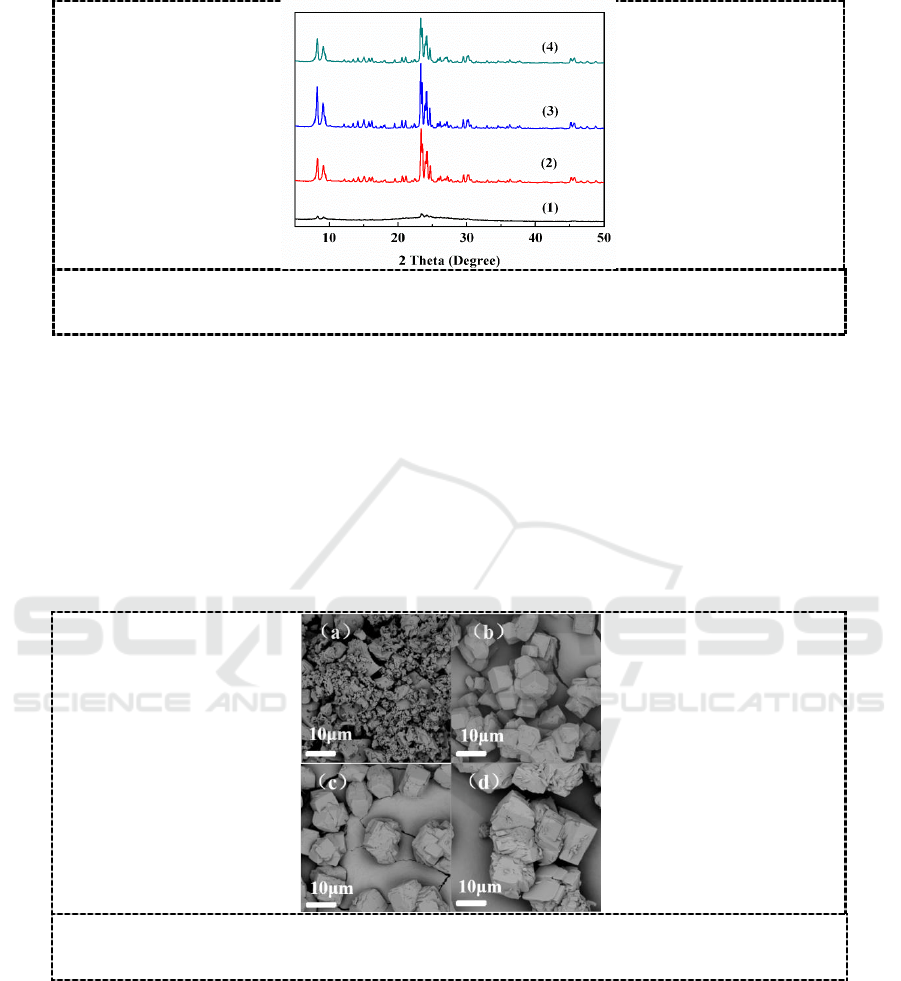
Figure 1. XRD patterns of the samples synthesized with different crystallization
temperature. (1) 160
o
C, (2) 170
o
C, (3) 180
o
C, (4) 190
o
C
2) SEM characterization results
Figure 2 shows the SEM images of the samples synthesized at different crystallization temperatures
for 24 h. At low temperature of 160
o
C, the most phase is amorphous, and it will disappear at higher
temperature of 170
o
C to 190
o
C. The cubic-like single crystals with smooth surface with the size
about 10 μm were observed when the temperature is 170°C and 180°C. In addition, these single
crystals were in form of highly dispersed at 180
o
C. It was also observed that higher synthesis
temperature (190
o
C) led to increasing of crystal sizes, whose average crystal size is more than 10μm.
Because the high crystallization temperature is beneficial for the fast nuclei formation and their
continuous aggregation [11].
Figure 2. SEM images of the samples synthesized with different crystallization
temperature. (a) 160
o
C, (b) 170
o
C, (c) 180
o
C, (d) 190
o
C
3.1.2 Effect of crystallization time
1) XRD characterization results
Figure 3 shows XRD patterns of the samples synthesized with different times at 180
o
C. When the
solid materials were heated for 8 h, the characteristic diffraction peaks of ZSM-5 at 2θ=7-9
o
, 22-25
o
began to appear, and the relative crystallinity was only 33.8%. When prolonged the crystallization
time to 16 h, the amorphous phase completely converted into highly crystalline ZSM-5, and the
relative crystallinity reached to the high value of 99.5%. With further increase in time, the intensity
of the diffraction peaks gradually decreased.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
332
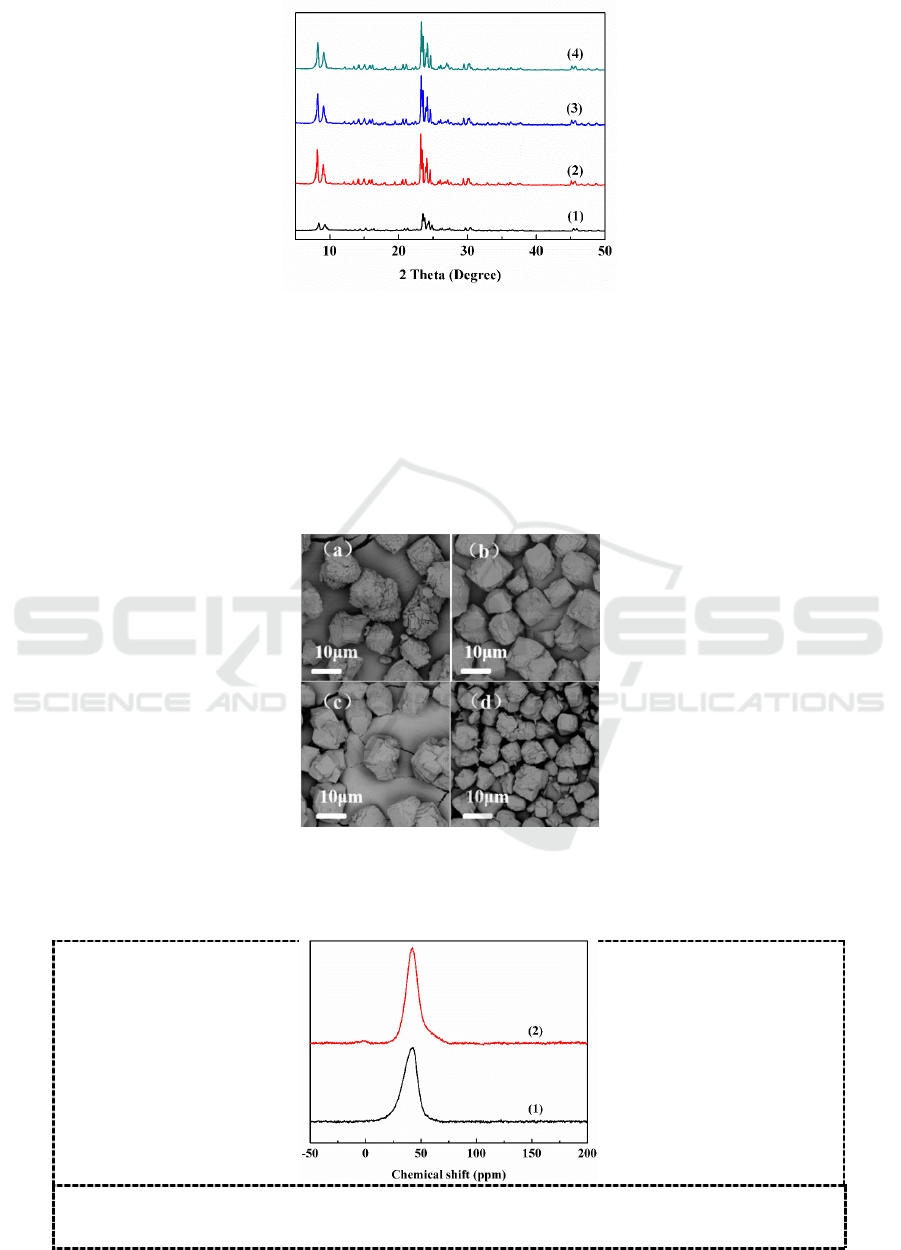
Figure 3.XRD patterns of the samples synthesized with different crystallization time. (1) 8h, (2) 16h,
(3) 24h, (4) 36h
2) SEM characterization results
Figure 4 shows the SEM images of the samples. At the short crystallization time of 8 h, the uniform
cubic-like morphology of crystals started to appear, but still was the aggregation of small particles.
When crystallization time was 16 h, the dispersed single crystals with smooth surface were obtained.
The particle size was about 8 μm and the crystallinity of ZSM-5 was 99.5%. As the crystallization
time prolonged, the morphology became irregular [12].
Figure 4.SEM images of the samples synthesized with different crystallization time. (a) 8 h, (b) 16 h,
(c) 24 h, (d) 36 h
3)
27
Al MAS NMR spectra
Figure 5.
27
Al MAS NMR spectra for the samples of (1) commercial zeolite and (2) S-
180-16 (1) 8h, (2) 16h, (3) 24h, (4) 36h
Synthesis of Single Crystal ZSM-5 by Solid-like State Conversion
333
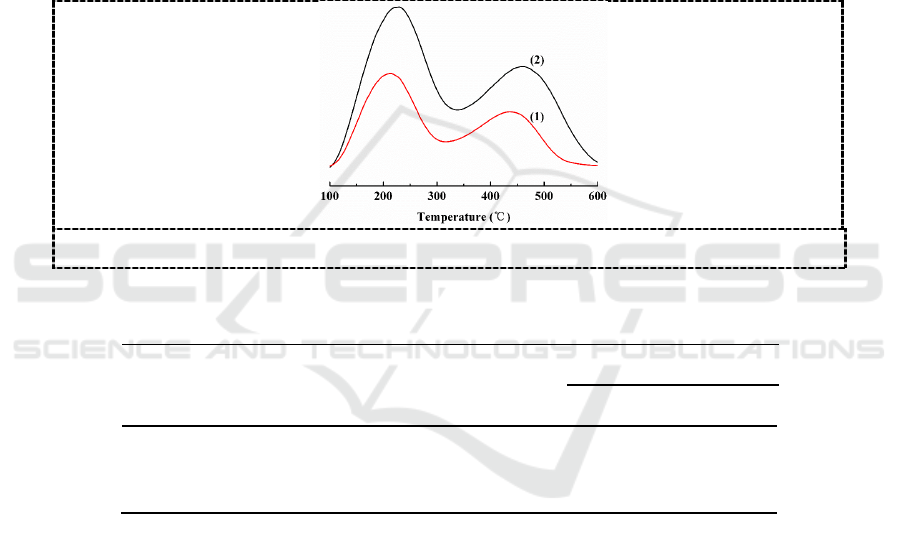
27
Al MAS NMR spectra of the sample S-180-16 and commercial ZSM-5 zeolite are shown in Figure 5.
The spectrum of all the samples exhibit a typical resonance peak at about 50 ppm, which are
assigned to Al tetrahedrally coordinated to oxygen (Al
IV
). The absence of resonance peak at 0 ppm
indicate that all of the Al species are incorporated into zeolite framework.
4) NH
3
-TPD acidity results
Figure 6 shows the NH
3
-TPD curves of the S-180-16 sample and the commercial ZSM-5 zeolite. All
samples showed two desorption peaks centered at 180-210
o
C and 420-500
o
C, which correspond to
weak and strong acid sites, respectively. The peak at the lower temperature is assigned to the
connection between H bonds and O–Si bonds of the weak acid center and non-acid center. The peak
at the higher temperature represents the strong acid sites. The area of peaks attributed to amounts
acid sites, which are given in Table 1. These results indicated that the sample S-180-16 has higher
acid strength and amounts than the commercial ZSM-5 zeolite.
Figure 6. TPD curves for the samples of (1) commercial zeolite and (2) S-180-16
Table 1.NH
3
-TPD acidity for the samples
Sample
n(SiO
2
)/n(Al
2
O
3
)
a
Acidity/mmol·g
-1
Weak
Strong
Total
Commercial ZSM-5 zeolite
55.2
0.32
0.17
0.49
S-180-16
52.8
0.59
0.34
0.93
a
Analyzed by XRF
3.2. Catalytic performance
Catalytic activity of above zeolite samples on toluene disproportionation is presented in Table 2. The
toluene conversion of S-180-16 sample is 40.18%, which is almost 15% higher than that of the
commercial ZSM-5 zeolite. And the selectivity towards p-xylene is 23.53% slightly higher than the
commercial ZSM-5 zeolite (22.30%), which goes well with acidity observed from NH3-TPD
experiments. The benzene to xylene (B/X) value of both zeolites is about 1, which is closed to the
thermodynamic balance [13].
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
334

Table 2.Catalytic properties of ZSM-5 for toluene disproportionation
Sample
X
T
a
(%)
S
PX
b
(%)
B/X
c
(mol / mol)
Commercial ZSM-5 zeolite
25.91
22.30
0.85
S-180-16
40.18
23.53
1.10
a
Conversion of toluene.
b
Selectivity to xylene.
c
molar ratio of benzene to xylene.
4. Conclusions
In summary, ZSM-5 single crystals have been synthesized via solid-like conversion. The crystals
were in cubic-like morphology with a size of 8μm. Furthermore, Al species were incorporated into
the zeolite framework and led to effective acid sites, resulting in a better catalytic performance than
commercial ZSM-5 zeolite.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21661031,
No. 21263026).
References
[1] Yue Y Y, Liu H Y, Yuan P, Li T S, Yu C Z, Bi H T and Bao X J 2014 J. Catal. 319 200
[2] Yue Y Y, Kang Y, Bai Y, Gu L L, Liu H Y, Bao J, Wang T H, Yuan P, Zhu H B, Bai Z S and
Bao X J 2018 Appl. Clay Sci. 158 177
[3] Meng X J and Xiao F S 2015 Prog. Chem. 27(5) 503
[4] Xu W Y, Dong J X, Li J P, Li J Q and Wu F 1990 J. Chem. Soc., Chem. Commun. 10(10) 755
[5] Weitkamp J, Hunger M, 2005 Stud. Surf. Sci. Catal. 155 1
[6] Luo W, Yang X Y, Wang Z R, Huang W F, Chen J Y, Jiang W, Wang L J, Cheng X W, Deng
Y H and Zhao D Y 2017 Microporous Mesoporous Mater. 243 112
[7] Ren L M, Wu Q M, Yang C G, Zhu L F, Li C J, Zhang P L, Zhang H Y, Meng X J and Xiao F
S 2012 J. Am. Chem. Soc. 134(37) 15173
[8] Pan F, Lu X C, Zhu Q S, Zhang Z M, Yan Y, Wang T Z and Chen S W 2014 J Mater. Chem. A
2(48) 20667
[9] Paris M, Bizot H, Emery J, Buzaré J, and Buléon A 1999 Carbohydr. Polym. 39(4) 327
[10] Kim J and Ahn W 1991 Appl. Catal. A-Gen. 71(1) 55
[11] Jiang J L, Duanmu C, Yang Y, Gu X and Chen J 2014 Powder Technol. 251(1) 9
[12] Pan F, Lu X C, Wang Y, Chen S W, Wang T Z and Yan Y 2014 Microporous Mesoporous
Mater. 184(1) 134
[13] Suganuma S, Nakamura K, Okuda A and Katada N 2017 J. Mol. Catal. 435 110
Synthesis of Single Crystal ZSM-5 by Solid-like State Conversion
335
