
Effect of a Modified SAF on Aluminate Hydration of Cement
H Y Wu, Y Q Jiang
*
and Y Wang
College of Mechanics and Materials, HoHai University, Nanjing 210098, China
Corresponding author and e-mail: Y Q Jiang, yqjiang@hhu.edu.cn
Abstract. Su lfonated acetone forma ldehyde (SAF) condensate type high range water reducer
was modified by grafting with lignosulfonate for the sake of mitigation bleeding of concrete.
Effect of the modified SAF (MSAF) on assemblage of aluminate hydrates in hardened cement
paste was investigated by XRD and SEM. Experimental results indicated that the MSAF
inhibits the hydration progress of C
3
A, reduces the intercalation between superplasticizer
mo lecules and aluminate hydrates and changes the dissolution-precipitation process of
limestone fille r b lend cement. Reaction between C
3
A and calcite is controlled by M SAF.
Consequently, monosulfoaluminate was still observed in the paste containing limestone filler
hydrated for 28 days, which was comparable with hydrates in control and polynaphthalene
sulphonate (PNS) dispersed pastes. Different in aluminate hydrates explains why MSAF or
MSAF-maleic type polycarbo xylate ad mixture (MSAF-PCA) comb ined superplasticizer is
more compatible with cements containing limestone filler than PNS.
1. Introduction
High range water reducers or superplasticizers are absolutely necessary for modern concrete mixture.
High performance concrete, which has been widely applied in engineering, is a successful example of
the combination of chemical admixtures and concrete technology [1-3]. Although the dosage of
superplasticizer is usually less than 1 wt. % of cement, it does play an important role in improving
homogenous characteristic, rheological property, strength and durability of cementitious materials [4,
5].
Polynaphthalene sulfonate (PNS) has been employed as high range water reducer in concrete for
more than thirty years. Application of PNS will be blocked for environmental issues of the large
consumption of industrial naphthalene which derives from coal tar, and for the incompatible problem
with cement blended with limestone filler [6]. There were also some limitations about the use of
polycarboxylate type admixtures (PCA) because of their high cost. Sulfonated acetone formaldehyde
(SAF) condensate based high water-reducer, one of dominant superplasticizers in the market, has
been widely developed in China for one decade. The advantages of SAF are high water reducing
efficiency, better compatibility with cements, mineral admixtures and other chemical admixtures,
lower application cost, and energy-saving production process (ie. no heat resource is needed). But,
there is a flaw in deed. Concrete or mortar superplasticized with SAF is prone to bleeding, which
results in red coloured surface. Although lots of researches have been done concerning upgrading the
performance of SAF in both experimental and theoretical aspects, little references are available for
industry production. Heat release of aldol reaction during synthesis of SAF at high pH (13-14)
solution makes it more difficult to graft SAF with most commonly used chemicals. Lignosulfonate,
378
Wu, H., Jiang, Y. and Wang, Y.
Effect of a Modified SAF on Aluminate Hydration of Cement.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 378-385
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

concentrated from black liquor, can be modified by oxidation and polymerization as high range water
reducer at similar conditions for SAF production [7]. The present study synthesized lignosulfonate
modified SAF (MSAF) by catalyzed condensation. Concrete added with MASF shows excellent
stability and viscosity, and better slump retention.
As is known, mechanism of condensate type water reducer is always linked with electrostatic
repulsion, and ettringite and AFm (monosulfoaluminate, monocarboaluminate, etc.) formation are
involved [8-10]. It seems that limestone filler favors crystallization of monocarboaluminate hydrate
rather than monosulfoaluminate. But the present research showed different assemblage of aluminate
hydrates in MSAF dispersed hardened cement paste, which implies fundamental mechanism of
cement-MSAF interaction should be explored, especially when limestone filler is used.
2. Experiment
2.1. Materials
Cement: A kind of Portland cement equivalent to Chinese P.II 52.5 containing 4 wt. % limestone
filler was employed. Chemical composition of cement is shown in Table 1. The mineral content of
C
3
S, C
2
S, C
3
A and C
4
AF are calculated by Bogue equation as 53.6%, 23.0%, 7.4% and 9.4% by mass
respectively.
Table 1. Chemical composition of cement [wt. %].
CaO
SiO
2
Al
2
O
3
Fe
2
O
3
MgO
SO
3
LOI
64.5
22.0
4.8
3.1
0.9
1.9
1.0
Chemicals: CaCO
3
, Al
2
O
3
, acetone, sodium sulfite, lignosulfoaluminate, formaldehyde solution
(wt. 37%), naphthalene (wt. 95%), oil of vitriol (wt. 98%), sodium hydrate solution (wt. 30%), lime
(wt. 86.2%), catalyst, and maleic anhydride type PCA. All of the chemicals were obtained from
Jiangsu Provincial general building materials research institute, China.
Water: Deionized water.
MSAF: The MSAF was synthesized following the progress: In a 1000 ml three-necked, round
bottomed flask equipped with a reflux condenser and a thermometer, 150 g lignosulfonate solution
with 30 wt. % concentration was added and modified by oxidation according to reference [7].
Temperature was cooled to 50 °C by cooling with cold water. 45 g sodium sulfite were dissolved in
197.1 ml DI water at 40°C by vigorous stirring. Then 40.6 g acetone was added. The reaction flask
was then kept at 40 °C for 1 h with constant stirring. After sulfonation, 123.3 g aqueous
formaldehyde of 37 wt. % concentration was gradually fed into the reactor through a dropping funnel.
When the temperature reached 60 °C ~65 °C , the fed of aqueous formaldehyde was pause and the
temperature was maintained for 1 h. Then, formaldehyde addition was continued, and temperature
was allowed to rise to 95 °C ±2 °C and the reaction continued for 3 h under catalysis. Finally, the
solution was cooled to ambient temperature. An aqueous, brown MSAF solution and a solids content
of 30 wt. % was obtained.
C
3
A: Pure tricalcium aluminate (C
3
A) was prepared via a sol-gel progress followed by calcination
of a 3:1 molar ratio of CaCO
3
and Al
2
O
3
for 6h at 1350°C . The progress was repeated until the
content of f-CaO was less than 0.5%.
2.2. Experimental method
XRD analyses: To begin with, hydration of C
3
A in the presence of various superplasticizers was
studied. For this purpose, C
3
A was hydrated in deionized water.1g C
3
A and superplasticizer were
added into 100ml deionized water. The suspension was stired at 20°C for 48h unedr argon,the
centrifuged and the precipited finally dried at 50°C for 48h. XRD patterns were obtained with a
Effect of a Modified SAF on Aluminate Hydration of Cement
379
BD90 diffractometer operating at 30kV and 30mA, using Cu-Kα tadiation at 0.02˚s
-1
between 5˚
and 40˚ 2θ angles.
SEM analyses: Cement paste with initial w/c = 0.29 and 1.5 % aqueous superplasticizer (the
combined superplasticizer comprises of 70 % MSAF and 30% PCA) on a mass basis was mixed at
20 °C ±2 °C in blender using the following protocol: 120 seconds of low speed mixing, a rest of 15
seconds while the sides of the mixing vessel were scraped down, and 120 seconds more of high speed
mixing to prepare the final product. Cast wafers of the prepared pastes, 30 mm in diameter and 5 mm
in thickness, were placed in small, capped copper vials. Then the vials were cured inside of a curing
box maintained at 20 °C ±2 °C and RH >90%. At 3 and 28 days, wafers were removed from their
vials and crushed into small pieces. Some of the small pieces (typically 2 to 3 mm in size) were
retained for SEM. After the water remaining in the small pieces was replaced by ethanol, the pieces
were dried in an oven at 60°C ~65°C . The specimens were aurum sprayed before SEM analysis and
were observed using Japanese JSM5610LV SEM analyzer.
3. Results and discussions
3.1. Hydration of C
3
A in the presence of MSAF
With the fastest hydration rate amoung cement miners, C
3
A has the biggest influence on the
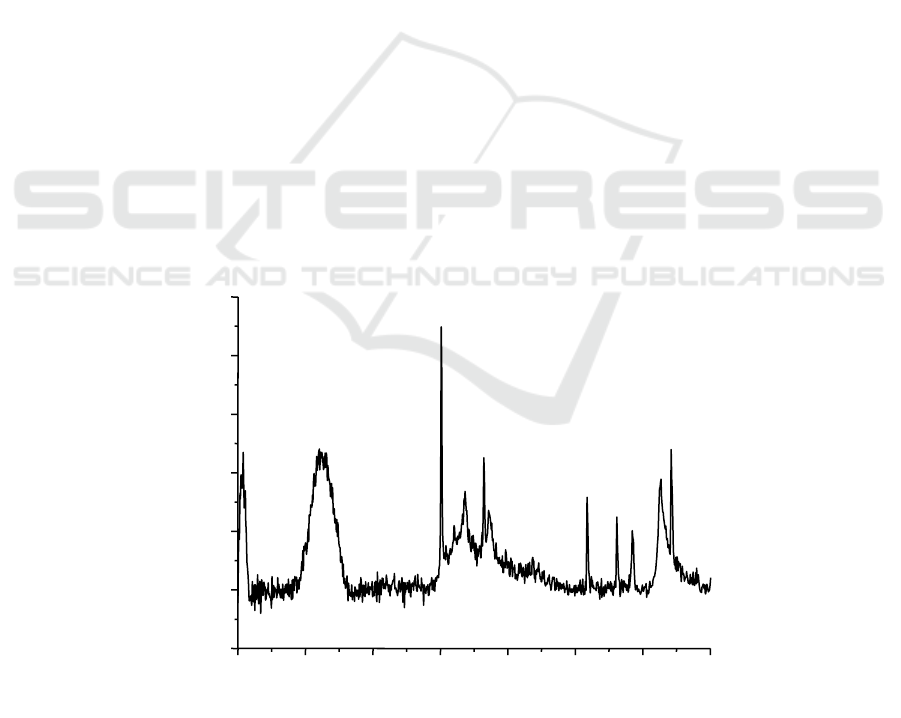
adaptability between the superplasticizer and cement. Figure 1, 2 and 3 shows the XRD spectrum of
C
3
A hydration products incorparating PNS, MSAF and MSAF-PCA, respectively. It was obvious
that the main hydration product of C
3
A were katoite and C
4
AH
13
while superplasticizer was added in.
Compare with the hydration products of C
3
A within PNS (Figure 1), C
3
A diffraction peak were
observed apparently in the XRD spectrum within MSAF (Figure 2) and PNS- MSAF (Figure 3),
which indicated that residual C
3
A were remained in hydration products. The result demonstrated that
the hydration of C
3
A would be restrained when the MSAF or MSAF-PCA was added in cement paste.
Figure 1. XRD parrern of the hydration products of C
3
A incorporating PNS (K=katoite, I=Inorganic
LDH, O=Organic LDH).
5
10
15
20
25
30
35
40
A.U.
2θ
O
, CuKα
O
K
C
4
AH
13
K
K
K
K
K
K
K— C
3
AH
6
I
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
380
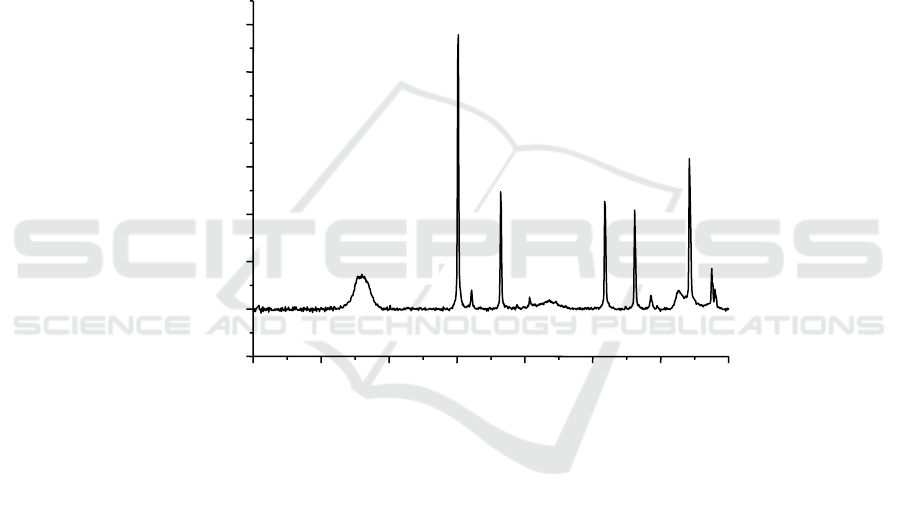
It was found that C
3
A hydrating in aqueous superplasticizer solution may partially incorporate the
anionic superplasticizer in between its [Ca
2
Al(OH)
6
]
+
main layers. The result is an organo-mineral
phase with layered structure similar to those of C
2
AH
8
and C
4
AH
13
, respectively. This could be
proved by the diffraction peak of organic layered double hydroxide(LDH) found in Figure 1 which
corresponded to the hydration products incorporating PNS, but the much weaker diffraction peak
could only found in the C
3
A hydration products incorporating MSAF(Figure 2), and barely exist in
the C
3
A hydration products incorporating MSAF-PCA(Figure 3). It shows that PNS molecules can
insert in C
3
A hydration products, and form Organic LDH phase, which on the contrary hardly exits in
C
3
A hydration products with MSAF or MSAF-PCA added in. Organic LDH phase existing in
hydration productions means the intercalation of superplasticizer molecules in the internal surface of
C
3
A hydration productions, which would cause the reduction of superplasticizer molecules affective
absorption on the surface of cement particles, consequently greatly weakened the beneficial effect of
superplasticizer.
Figure 2. XRD parrern of the hydration products of C
3
A incorporating MASF (K=katoite,
I=Inorganic LDH, O=Organic LDH).
5
10
15
20
25
30
35
40
K
Al(OH)
3
K
K
K
K
C
3
A
A.U.
2θ
O
, CuKα
Ca
4
Al
2
O
6
(CO
3
)
0.5
(OH)11.5H
2
O
K —C
3
AH
6
O
Effect of a Modified SAF on Aluminate Hydration of Cement
381
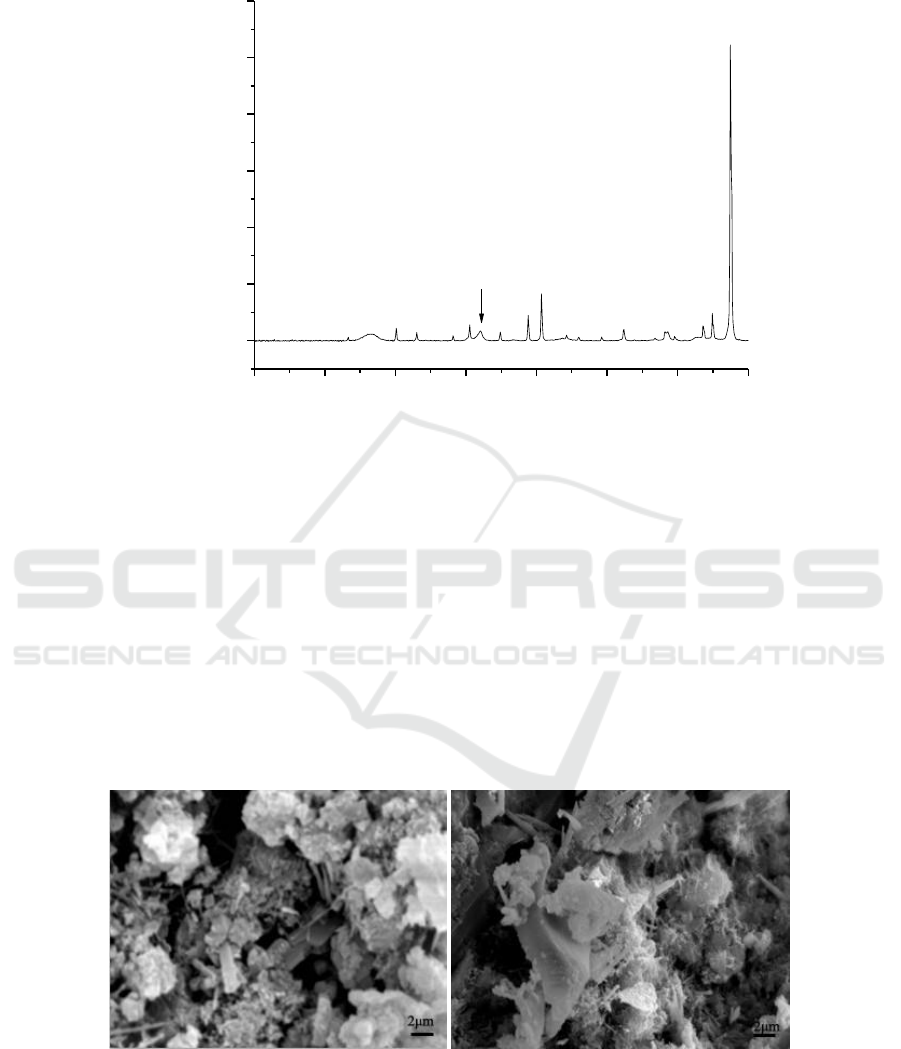
Figure 3. XRD parrern of the hydration products of C
3
A incorporating MSAF-PC (K=katoite,
I=Inorganic LDH, O=Organic LDH).
3.2. Morphology of aluminate hydrates
The morphology of hydrate assemblage of cement pastes with limestone was showed in Figure 4-a.
Ill-crystallized and nearly amorphous character of C-S-H, thin needle-like ettringite crystals shape of
flocculation-like and cubic calcite were observed in Figure 4-a(left). Because of the reaction between
aluminate and the limestone, the hydration products of aluminate tend to be monocarbonate rather
than monosulfate, which could be proved by the phases shaped as sheet-like crystals in Figure 4-
a(right). As it reported, the calcite would act as a preferential substrate for the germination and
growth of hydration products, thus accelerating the hydration progress, the C-S-H and needle-like
ettringite crystals around calcite grains found in 28d SEM picture could support this idea.
Blank: 3d (left), 28d (right)
(a)
5 10 15 20 25 30 35 40
rel.Intensity
Angles 2θ (degrees) CuKα
O
I
C3A
K
C4AH13
C3A
C3A
K
K
K
K
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
382

PNS: 3d (left), 28d (right)
(b)
MSAF: 3d (left), 28d (right)
(c)
MSAF-PCA: 3d (left), 28d (right)
(d)
Figure 4. Microstructure of aluminate hydrates of cement pastes with and without superplasticizer.
Figure 4-b, Figure 4-c and Figure 4-d corresponded to the pastes incorporating PNS, MSAF and
MSAF-PCA respectively. Ettringite that crystallized in the presence of superplasticizer, especially
the MSAF and MSAF-PCA, were found to have a morphology quite different from the ones observed
in the absence of superplasticizer. In the presence of superplasticizer, ettringite crystals lost its
needle-like shape to form rather different crystals. Ettringite crystals keep its needle-like but thinner
and longer in the presence of PNS. The typical morphology of ettringite crystals were hardly found at
the early age of hydration in the cement paste incorporated MSAF and MSAF-PCA. Some block-like
Effect of a Modified SAF on Aluminate Hydration of Cement
383

and cluster-like AFt phases which shape contrasts with the long needle-like ones observed in the
absence of superplasticizer were found in the cement paste incorporated MSAF. And the amounts of
ettringite crystals were much fewer compared with ones observed in the absence of superplasticizer,
in other words, could retard or even restrain the formation of ettringite crystals at the early time.
Compared with the cement paste without MSAF or MSAF-PCA, the sheet-like crystals of
monocarboaluminate were hardly found the SEM picture. In the pastes incorporating MSAF, many
needle-like crystals of ettringite and hexagonal crystals of monosulfoaluminate was still observed in
the paste containing limestone filler hydrated for 28 days (Figure 4-d right), which was comparable
with hydrates in control and polynaphthalene sulphonate (PNS) dispersed pastes. It was indicated that
the reaction between C
3
A and calcite is restricted. Ettringite and calcium monosulfoaluminate, other
than ettringite and calcium monocarboaluminate or hemicarboaluminate, are coexisting in hardened
cement paste blended with limestone filler and dispersed by MSAF or MSAF-PCA combined
superplasticizer.
4. Conclusions
According to the analysis and discussion, conclusions are drawn as follows:
Sulfonated acetone formaldehyde condensate superplasticizer has been modified by catalyzed
condensation with lignosulfonate. Drawbacks of SAF, promotion bleeding of and putting colour on
cementitious materials, were overcome by the modification. Dissolution-precipitation process of C
3
A
at early age may be controlled by MSAF to some extent which results in little intercalation of
superplasticizer into Ca-Al-LDH and more ettringite formation at initial stage and dormant period of
cement hydration. Consequently, unhydrated C
3
A reacts with ettringite to form calcium
monosulfoaluminat via topochemical reaction at later age. As such, MSAF changes the assemblage
of C
3
A-CaSO
4
-CaCO
3
hydrates. Reaction between C
3
A and calcite is restricted. Ettringite and
calcium monosulfoaluminate, other than ettringite and calcium monocarboaluminate or
hemicarboaluminate, are coexisting in hardened cement paste blended with limestone filler and
dispersed by MSAF or MSAF-PCA combined superplasticizer. This explains how MSAF modifies
the stability of fresh cementitious materials and why MSAF is more compatible with cements
containing limestone filler than PNS.
Further investigation should be down to clarify action mechanism of MSAF for better
understanding C
3
A-MSAF interaction and reasonably use of MSAF.
Acknowledgment
The authors gratefully acknowledge partial supports for this research which have come from Key
Program of National Natural Science Foundation of China (Grant number: 51738003).
References
[1] Jin L, Song W, Shu X and Huang B 2018 Constr. Build. Mater. 159 690-694
[2] Li P, Yu Q and Brouwers H 2017 Constr. Build. Mater. 153 740-750
[3] Huang F, Li H, Yi Z, Wang Z and Xie Y 2018 Constr. Build. Mater. 166 833-838
[4] Akhlaghi O, Aytas T, Tatli B, Sezer D, Hodaei A and Favier A 2017 Cem. Concr. Res. 101
114-122
[5] Huang H, Qian C, Zhao F, Qu J, Guo J and Danzinger M 2016 Constr. Build. Mater. 110 293-
299
[6] Jiang Y, Zhang S and Damidot D 2010 J. Southeast Univ. 26 574
[7] Yu W, Xie X, Li Y, Li Y, Chen R and Qiu X 2016 J. Mater. Chem. C 4 34
[8] Dalas F, Pourchet S, Rinaldi D,Nonat A, Sabio S and Mosquet M 2015 Cem. Concr. Res. 69
105-113.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
384

[9] Shi C, Zhang G, He T and Li Y 2016 Constr. Build. Mater. 112 261-266
[10] Holanda F, Schmidt H and Quarcioni V 2017 Cem. Concr. Compo. 83 384-393
Effect of a Modified SAF on Aluminate Hydration of Cement
385
