
Anti - caking KAl (SO
4
)
2
12H
2
O - MgSO
4
7H
2
O Composite
for Thermal Storage Material by Addition of Al
2
O
3
Powder
M Zheng
*
, Z Yang and J L Shi
School of Chemical Engineering, Northwest University, Xián, 710069, China
Corresponding author and e-mail: M Zheng, mszheng2@yahoo.com
Abstract. Alumina powder is added as anti- caking agent to prevent the agglomeration of
phase transition thermal storage composite KAl (SO
4
)
2
·12H
2
O - MgSO
4
·7H
2
O with the ratio
of 2:8, heating and cooling cycle test is conducted to characterize its cyclic properties. The
results show that the mixed salt system with alumina anti- caking agent is with good thermal
storage property. The exothermic enthalpy of phase transition is still kept at 188.5J / g after
200 cycles, super-cooling of average 2C, stable exothermic phase transition temperature of
average 47 C and longer duration of exothermic platform of average 7.2 min. It is
demonstrated that the alumina powder is active in preventing the mixed salt thermal storage
material from agglomeration.
1. Introduction
Caking is a common phenomenon for material from a loose state to dense one [1]. Most inorganic
salt product behaves this caking phenomenon [2], this feature changes their original property and
causes inconvenience sometimes.
In the study of cyclic properties of hydrated inorganic salt phase change materials, it found that
some thermal storage materials also produce agglomeration after several thermal cycles, thus
affecting the material's heat storage performance. In order to improve this feature, anti- caking agents
are often added to the material to prevent the hydrated inorganic salt from agglomerating.
Currently, the common mechanism for crystal agglomeration is the crystalline bridging theory and
capillary adsorption theory proposed by Gamondes in 1977 [3]. The agglomeration theory suggests
that the agglomeration of the crystal occurs after the surface is dissolved and re-crystallized; the
small crystal grains fuse into clusters afterword. Therefore, the inert anti- caking agent is inserted
into the crystal particles so as to form a barrier and prevent formation of the crystal bridge during the
dissolution and re-crystallization. Thus the agglomeration is easily broken. Arno A. C. Bode et al
studied the effect of anti- caking agent on sodium chloride [4], Stefan Baueregger et al analyzed the
effect of inert powder kaolin on latex polymer film forming [5].
Phase change material could lose its ability of thermal storage due to the formation of caking. On
the other hand, when the inert anti- caking agent is added to inorganic salt, it will hinder the
crystallization process, and thus affecting its heat storage performance as well. Therefore, suitable
Zheng, M., Yang, Z. and Shi, J.
Anti - Caking Kal (SO4)2.12H2O - MgSO4.7H2O Composite for Thermal Storage Material by Addition of Al2O3 Powder.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 403-408
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All r ights reserved
403

inert anti- caking agent and its amount should be selected appropriately, so as to optimize the PCM
thermal storage properties.
KAl (SO
4
)
2
12H
2
O-MgSO
4
7H
2
O mixed hydrated inorganic salt system is a group of thermal
storage material with advantages of high energy density, cheap and easily to conduct preparation. As
to the mass ratio of KAl(SO
4
)
2
12H
2
O to MgSO
4
7H
2
O being 2 to 8, it has the characteristics of high
latent heat of phase change, good thermal conductivity, low degree of super-cooling (about 1 °C ),
and no segregation [6]. The phase change temperature of the material is stable at about 48 °C after
several cycles of cooling and heating, which is a good hydrated salt phase change heat storage
material suitable for low temperature solar air - drying system. However, this material suffers from
agglomerate when circulated to about 20 times, and the dense agglomeration increases gradually with
the cyclic number.
The aim of the present paper is to prevent the material with the mass ratio of KAl(SO
4
)
2
12H
2
O to
MgSO
4
7H
2
O being 2 to 8 from agglomeration, alumina powder is added as an anti- caking agent to
perform the work.
2. Preparation and basic test
2.1. Experimental material
The main chemicals are: KAl (SO
4
)
2
12H
2
O, Zhengzhou Paiey Chemical Reagent, Zhengzhou, purity
of 99.5%; MgSO
4
7H
2
O, Tianli Chemical Reagent Co. Ltd., Tianjin, purity of 99%; Al
2
O
3
powder,
Xi’an Chemical Reagent, Xi’an, purity of 99.5%.
2.2. Laboratory equipment
The experimental equipments include: HCT-1 differential scanning calorimetry balance, DZF-6030
vacuum oven; DF-101S constant temperature heater with magnetic stirrer; a thermometer (apuhua
TM-902C, -50 °C ~ 1300 °C , accuracy 0.1 °C ); JJ124BC electronic balance (Max = 120g, Min = 20d,
d = 0.1mg).
2.3. Characteristics of thermal properties of MgSO
4
7H
2
O and KAl (SO
4
)
2
12H
2
O components
The experimental procedure for DTA and step-cooling curve tests are described as those in [6].
As to step-cooling curve, temperature data is recorded every 10s for once; step cooling curve is
drawn after the cooling temperature tests. While for DTA test, the temperature ranges from room
temperature to 150°C with heating rate of 1 °C / min.
While the DTA curve and the step cooling curve of KAl (SO
4
)
2
12H
2
O are shown in [6]. The
DTA curve shows a single endothermic peak at 75.7 °C , which exhibits a large latent heat of
882.58kJ/kg.
The step cooling curve of KAl (SO
4
)
2
12H
2
O represents that the crystallization of the molten of
KAl (SO
4
)
2
12H
2
O begins at 63.0°C followed by a temperature rising to 73.4°C , which is because of
the releasing of latent heat as well, and implies a relative bigger super-cooling of 10.4°C .
The experimental DTA curve and step cooling curve for MgSO
4
7H
2
O are shown in [7]
respectively.
From DTA curve of MgSO
4
7H
2
O, it can be seen that MgSO
4
7H
2
O exhibits three endothermic
peaks during heating process at 46.1 °C , 81.4°C and 106.4 °C , respectively. The total phase change
latent heat is 811.97kJ/kg, which is phase change material with high latent heat.
The step cooling curve of MgSO
4
7H
2
O shows that the molten MgSO
4
7H
2
O begins to crystallize
at 51.8 °C followed by a temperature rising to 60.4°C , which is owing to the releasing of latent heat,
and indicates a super-cooling of 8.6 °C .
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
404
3. Preparation of anti - caking phase change heat storage composite material and its property
test
3.1. Preparation of anti - caking phase change heat storage composite material
The preparation process is as follows,
(1) The milled KAl (SO
4
)
2
12H
2
O and MgSO
4
7H
2
O powders are mixed and poured into a
2050ml plastic test tube, the mass ratio is 2:8. The total amount is 10g; then, the milled anti-caking
agent Al
2
O
3
of 0.1 g is poured into the test tube as well.
(2) The test tube is placed in the thermostat heating magnetic stirrer with a constant temperature
heater, and kept half an hour after the material fully melting;
(3) The test tube is removed from the thermostat heating magnetic stirrer and stood at room
temperature, the temperature data is recorded every 10s, and then a step cooling curve could be
drawn after the tests.
3.2. Step cooling curve analysis of anti - caking composite materials
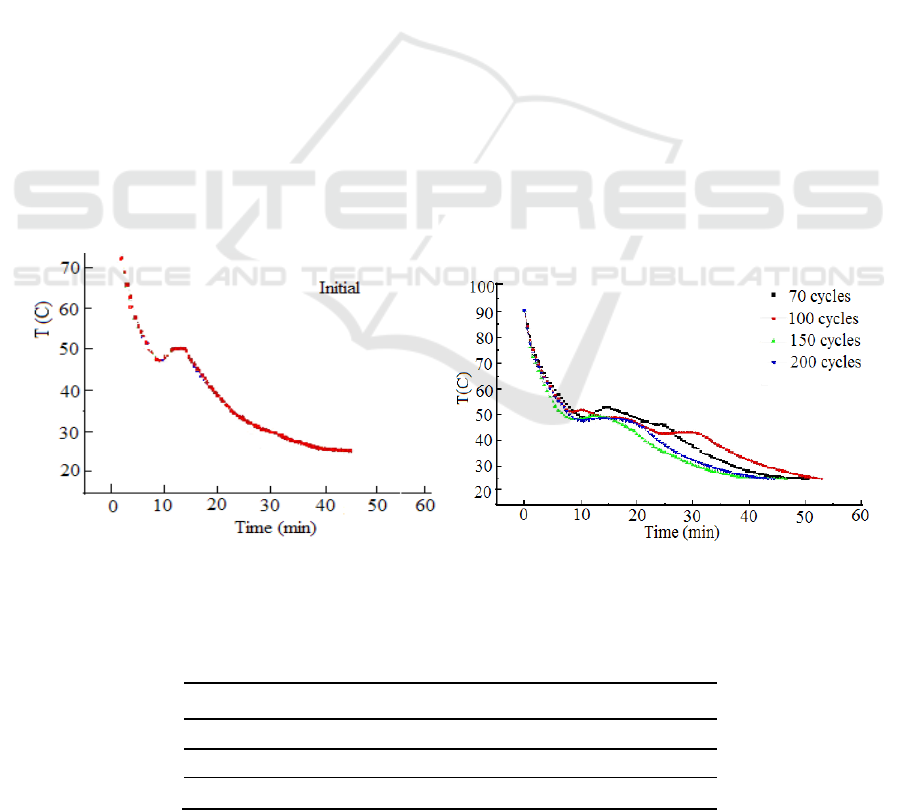
Shown in figure 1 is the initial step - cooling curve of the composite, while figure 2 is the step -
cooling curve of the composite suffering 70th, 100th, 150th, 200th cyclic heating-cooling tests. The
shapes of the step - cooling curves of the composite are different from those of their components,
says, MgSO
4
7H
2
O and KAl (SO
4
)
2
12H
2
O, but a little bit like that of MgSO
4
7H
2
O because more
MgSO
4
7H
2
O is contained in the composite. The tested solidifying temperature T
c
, the duration of
exothermic platform t
p
and super-cooling T
sc
from Figures 1 and 2 are listed in Table 1.
As can be seen from table 1, the solidifying temperature T
c,
the duration of exothermic platform t
p
and super-cooling T
sc
of this anti- caking composite keep almost unchanged during cyclic heating-
cooling process.
Figure 1.step cooling curve of the composite
Figure 2.step cooling curve vs cyclic number
Table 1.the solidifying temperature T
c
, platform t
p
and
super-cooling T
sc
No. of cycling
initial
70
100
150
200
T
c
(°C )
46
48
43
48
49
t
p
(min)
6
10
7
7
8
T
sc
(°C )
4
4
0.4
1
0.8
Anti - Caking Kal (SO4)2.12H2O - MgSO4.7H2O Composite for Thermal Storage Material by Addition of Al2O3 Powder
405
3.3. DTA Analysis of MgSO
4
7H
2
O - KAl (SO
4
)
2
12H
2
O anti - caking composite
Take about 10mg anti - caking composite samples of KAl (SO
4
)
2
12H
2
O - MgSO
4
7H
2
O to conduct
their DTA test, the temperature ranges from room temperature to 150°C with heating rate of 1 °C
/min.
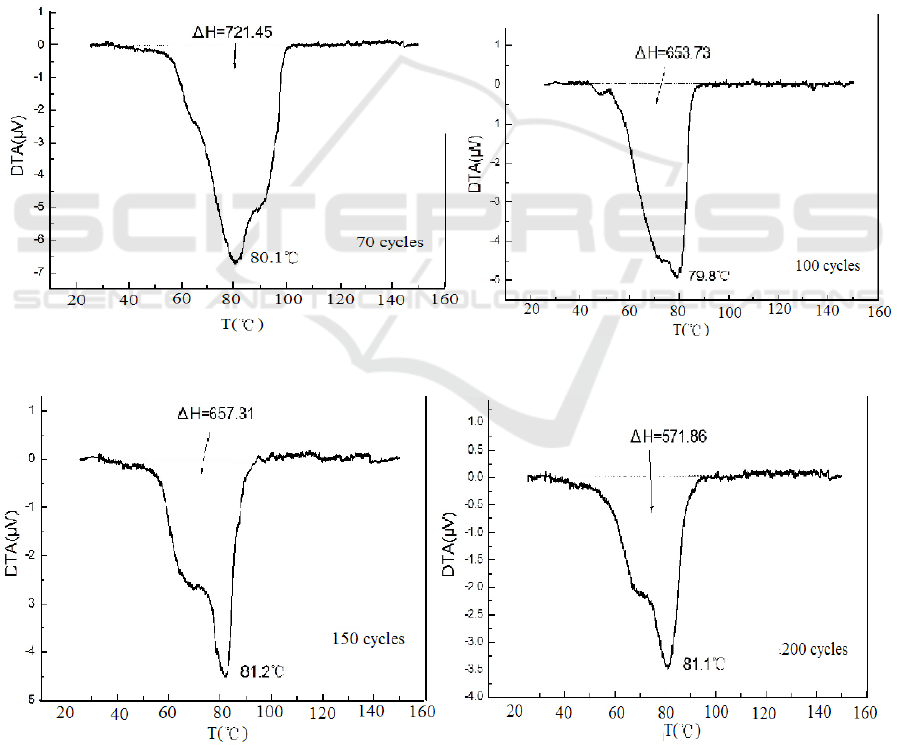
Figure 3 shows the DTA curves of the anti- caking composite after 70 and 100 heating – cooling
circles.
While figure 4 shows the DTA curves of the anti- caking composite after 150 and 200 heating –
cooling circles. Table 2 shows the thermal properties of this material by DTA test after some cycles.
The shapes of the DTA curves of the composite are different from those of their components, says,
MgSO
4
7H
2
O and KAl (SO
4
)
2
12H
2
O, maybe some metamorphosis is concerned.
Compared Figures 3 and 4 as well as table 2, it can be seen that the thermal properties of this anti
- caking composite keep stable, which implies that the interaction of the various components make
the composite stable.
Figure 3.DTA curves of the anti - caking composite after 70 and 100 heating – cooling cycles
Figure 4.DTA curves of the anti - caking composite after 150 and 200 heating – cooling cycles
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
406

Table 2.Thermal properties of this material by DTA test after some cycles
No. of cycling
initial
70
100
150
200
H (kJ/kg)
702.41
721.45
653.73
657.31
571.86
DTA peak (°C )
80.1
80.1
79.8
81.2
81.1
3.4. Heat release test of the anti - caking MgSO
4
7H
2
O - KAl(SO
4
)
2
12H
2
O composite
Take about 3g composite samples of KAl (SO
4
)
2
12H
2
O - MgSO
4
7H
2
O into a sealed tube to conduct
their heat release test. The sealed tube is warmed to 80 degree and kept for 30min. Thereafter, the
warmed sealed tube is put into an adiabatic vessel with 30 g water to measure the change of
temperature, and then compute the heat release amount of the composite.
Table 3 shows the heat release amount of the composite material after some cycles.
Table 3.heat release amount of the composite material after some cycles
No. of cycling
initial
70
100
150
200
W (kJ/kg)
204.1
211.6
205.2
194.7
188.5
Compared the data in table 2, it can be seen that the change of heat release amount of the
composite material after 200 cycles is not so significant.
4. Conclusions
(1) The anti - caking agent Al
2
O
3
is effective for KAl (SO
4
)
2
·12H
2
O - MgSO
4
·7H
2
O composite.
(2) The solidifying temperature T
c
, the duration of exothermic platform t
p
and super-cooling T
sc
of
this anti - caking composite keep almost unchanged during cyclic heating-cooling process.
(3) The thermal properties of this anti - caking composite keep stable.
(4) The change of heat release amount of the anti - caking composite material after 200 circles is
not so significant.
Acknowledgements
This work was financially supported by the Shaanxi Industrial Key Project (2016GY-155).
References
[1] Liu B F 1993 Caking and Prevention of Inorganic Salt, Shanxi Chem. Eng 2 20-22
[2] Yang Z L, Bao C G and Yan Z Y 1994 Effect of Surface Active Agent on Caking, Yunnan
Chem. Eng. 3 25-30
[3] Gamondes J P and Van’t Hoff J B 1977 How to Prevent Fertilizer Caking Nitorgen 105 32
[4] Bode Arno A C, Melvin V, Martin J 2015 Influence of Anti- caking agents on the Caking of
Sodium Chloride at the Powder and Two-crystal Scale Powder Technology 277 262-267
[5] Stefan B A, Margarita P and Johann P 2014 Influence of Anti-Caking Agent Kaolin on Film
Formation of Ethylene-vinylacetate and Carboxylated Styrene-butadiene latex polymers,
Cement and Concrete Research 58 112-120
[6] Zheng M, Luo J W, Zhang Y H and Chen P 2017 Preparation and characterization of
composite material Na
2
SO
4
·10H
2
O - KAl(SO
4
)
2
·12H
2
O for thermal storage, 1st
International Conference on New Material and Chemical Industry (NMCI2016), IOP Conf.
Series: Materials Science and Engineering 167 012007 doi:10.1088/1757-
Anti - Caking Kal (SO4)2.12H2O - MgSO4.7H2O Composite for Thermal Storage Material by Addition of Al2O3 Powder
407

899X/167/1/012007.
[7] Zheng M, Luo J W, Zhang Y H and Long Y P 2016 Preparation and characterization of
composite of MgSO
4
·7H
2
O and KAl (SO
4
)
2
·12H
2
O thermal storage material, Proceedings
of the 6th International Conference on Mechatronics, Materials, Biotechnology and
Environment (ICMMBE 2016), doi:10.2991/icmmbe-16.2016.55. 2016: 280-285
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
408
