
Effects of Hydrogen Bond Interaction on the Miscibility of
Poly (D, L-lactide) Composites Materials
Y Chai, Y F Song and P Y Zhang
*
Institute of Fine Chemistry and Engineering, College of Chemistry and Chemical
Engineering, Henan University, Kaifeng 475001, China
Corresponding author and e-mail: P Y Zhang, zhangpuyu@henu.edu.cn
Abstract. Blend materials of poly (D, L-lactide)/ poly (vinyl alcohol) (PDLLA/PVA), poly
(D, L-lactide)/poly (ethylene glycol)
300
(PDLLA/PEG
300
) and poly (D, L-lactide)/poly(ε-
caprolactone) (PDLLA/PCL), obtained by solvent-casting, have been studied by differential
scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FT-IR). DSC
results showed that PDLLA/PVA and PDLLA/PEG
300
are miscible in entire composition
range and PDLLA/PCL blends are immiscible. FT-IR results indicated that there are
hydrogen bond interactions in these blends except PDLLA/PCL. The miscibility of blends is
enhanced; however, the plasticizing role is not affected by hydrogen bond interaction through
hydroxyl end groups. With the increase of PEG
300
, the plasticizing role become obvious in
PDLLA/PEG
300
. The effects of hydrogen bond interaction between poly (D, L-lactide) and
other polymer chain were studied, the effects of the mixed temperature and solvent of the
blends were also discussed.
1. Introduction
Generally, synthetic polymers from petrochemical products were not easily degraded in the
environment. In recent years, biodegradable polymers attracted great interest for their environmental
friendly application [1-3]. The most popular and important biodegradable polymers were aliphatic
polyesters, such as polylactide (PLA), poly (glycolic acid) (PGA), poly (ε-caprolactone) (PCL) and
poly (3-hydroxy butyrate) (PHB). Poly (lactide) was thermoplastic polyester that was studied
extensively owing to its source accessible, non-toxic, easy processing, biodegradable,
biocompatibility and excellent thermal/mechanical properties, which was known as the most
promising biodegradable material [4-6]. Currently, PLA has already been applied in the plastic
industry, biomedical, pharmaceutical and other fields. Their applications mainly included drug
delivery systems, artificial organs, surgical devices, biosensors, materials for orthopedics and
biodegradable packaging [7-11].
PDLLA was a sort of amorphous polyester with a T
g
varied from around 50 to 60°C and a wide
range of melting temperatures, generally depending on its molecular weight and distribution [12].
But there were still a lot of restrictions due to its crisp and heat instability. To overcome these
drawbacks, many approaches have been investigated, and most excellent PLA materials were
prepared by copolymerization and blending [13-16]. Miscibility, mechanical properties and
416
Chai, Y., Song, Y. and Zhang, P.
Effects of Hydrogen Bond Interaction on the Miscibility of Poly (D, L-lactide) Composites Materials.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 416-424
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
crystallization of PLA blends have been studied in several papers [17-21], and it was considered that
PLA is immiscibility with other polymers many times.
Zhang [22] studied the miscibility of poly (D, L-lactide)/poly (vinylphenol) (PDLLA/PVPh)
blend, finding phase separation in blends over a wide composition. In contrast, Owen [23] indicated
the blends were complete miscibility with solution/precipitation and higher temperature. On the other
hand, some blends which were known as as-prepared before may be improved the miscibility by
using different blending methods and temperatures.
Recently, many investigations haven been also performed to enhance the impact resistance PLA
and compete with low cost commodity polymers. In this study, PVA, PEG
300
and PCL were using to
blending with PDLLA (Figure 1), and blends of poly (D, L-lactide)/poly (vinyl alcohol)
(PDLLA/PVA), poly (D, L-lactide)/poly (ethylene glycol)
300
(PDLLA/PEG
300
) and poly (D, L-
lactide)/poly(ε-caprolactone) (PDLLA/PCL) obtained by solvent-casting were analyzed by
differential scanning calorimeter (DSC) and Fourier transform infrared spectroscopy (FT-IR). The
changes of T
g
were investigated, and the miscibility and hydrogen bonds of blends have been also
discussed.
Figure 1. Chemical structures of PLA, PVA, PEG and PCL.
2. Experimental
2.1. Materials
PDLLA was synthesized in the way similar to that described in the literature [24-26]. PCL was
obtained by ring-opening polymerization of ε-CL using tin octoate as catalyst at 160°C . Both
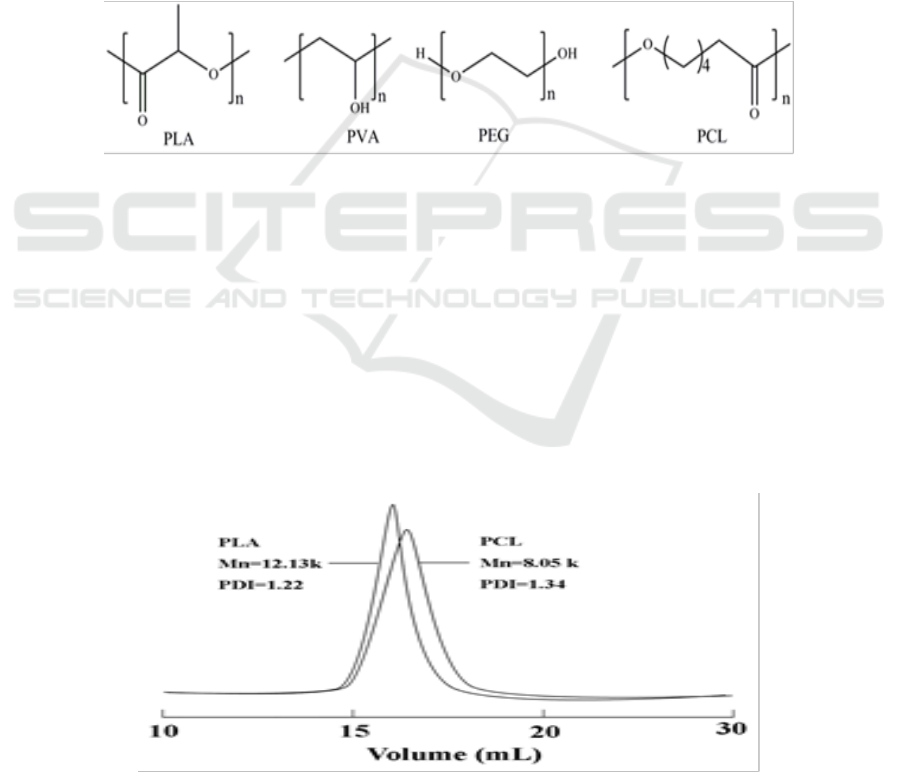
PDLLA and PCL were purified as literature described [27]. The molecular weight and molecular
weight distribution was measured at 25°C by gel permeation chromatography (GPC) with
tetrahydrofuran (THF) as eluent (1.0 mL/min) using multi-angle laser light scattering and refractive
index (Figure 2). PVA (Mn=6.97×104, % hydrolyzed) and PEG
300
(Mn =285~310) were supplied by
Sigma-Aldrich. Polymers were dried in a vacuum oven at 40°C over night previous use, and all other
reagents were used as received.
Figure 2. GPC profiles of PDLLA (Mn=121 300, PDI=1.22) and PCL (Mn=80 500, PDI=1.34).
Effects of Hydrogen Bond Interaction on the Miscibility of Poly (D, L-lactide) Composites Materials
417

2.2. Preparation of blends
PDLLA/PEG
300
were mixed in the desired amounts then dissolved in THF at room temperature. After
dissolved completely (about 12 h), the solution was cast on glass slide, solvent were volatilized for
48 h, then the blend film was dried in vacuum oven at 40°C for 48 h. PDLLA/PCL blend prepared in
a similar way. PDLLA/ PVA were dissolved in dimethyl formamide (DMF) at 140°C for 12 h, then
the solvent was allowed to evaporate at 100°C for 48 h; the films were dried in a vacuum oven at
60°C for 96 h.
2.3. Differential scanning calorimeter
Thermal analysis of the blend samples was performed using Mettler Instrument, model DSC822e.
The instrument was calibrated using indium and all experiments were carried out under a nitrogen
atmosphere. Approximately 5~6 mg of each blend was weighed and sealed in an aluminum pan. All
specimens were heated to 200°C at a rate of 100 K/min and held for 5 min to eliminate the thermal
history, followed by cooling to 20°C at a rate of 10 K/min. Then two consecutive scans were
performed with the scan rate of 10 K/min. The midpoints of heat capacity change in the DSC thermal
diagram obtained in the second heating run were taken as the glass transition temperature (T
g
).
2.4. Infrared spectroscopy
Infrared spectra of blends were recorded on a Nicolet AVATER 370 Fourier transform infrared
spectrophotometer (FTIR) at room temperature. A small quantity of sample was mixed with KBr,
carefully ground in a mill, and pressed to a pellet. The absorbance of all the studied samples was
within the absorbance range in which the Lambert-Beer law is obeyed. Second-derivative spectra
were smoothed with a quartic 15-point Savitzky-Golay smoothing filter. Care was taken on the
degree of distortion introduced by the smoothing algorithm, which was checked according to the
procedure reported elsewhere.
3. Results and discussion
3.1. DSC analysis
DSC is a well-known method to study the miscibility of polymer composite, based on the criterion of
a single glass transition temperature (T
g
) intermediate between those of pure polymers [28-32]. The
definition for miscible and partially miscible blends has been well established. There was usually
only one T
g
will appear in DSC thermograms at an intermediate temperature compared to that of the
T
g
value of each pure polymer if components were miscible. The single T
g
of blend should obey the
Fox equation describing the relationship between the T
g
value, of the blend and its composition, as
well as T
g
values of components in the pure state. The T
g
value of each component phase should be
affected by the other one, even if two components were only partially miscible, and it was usually
depended on composition.
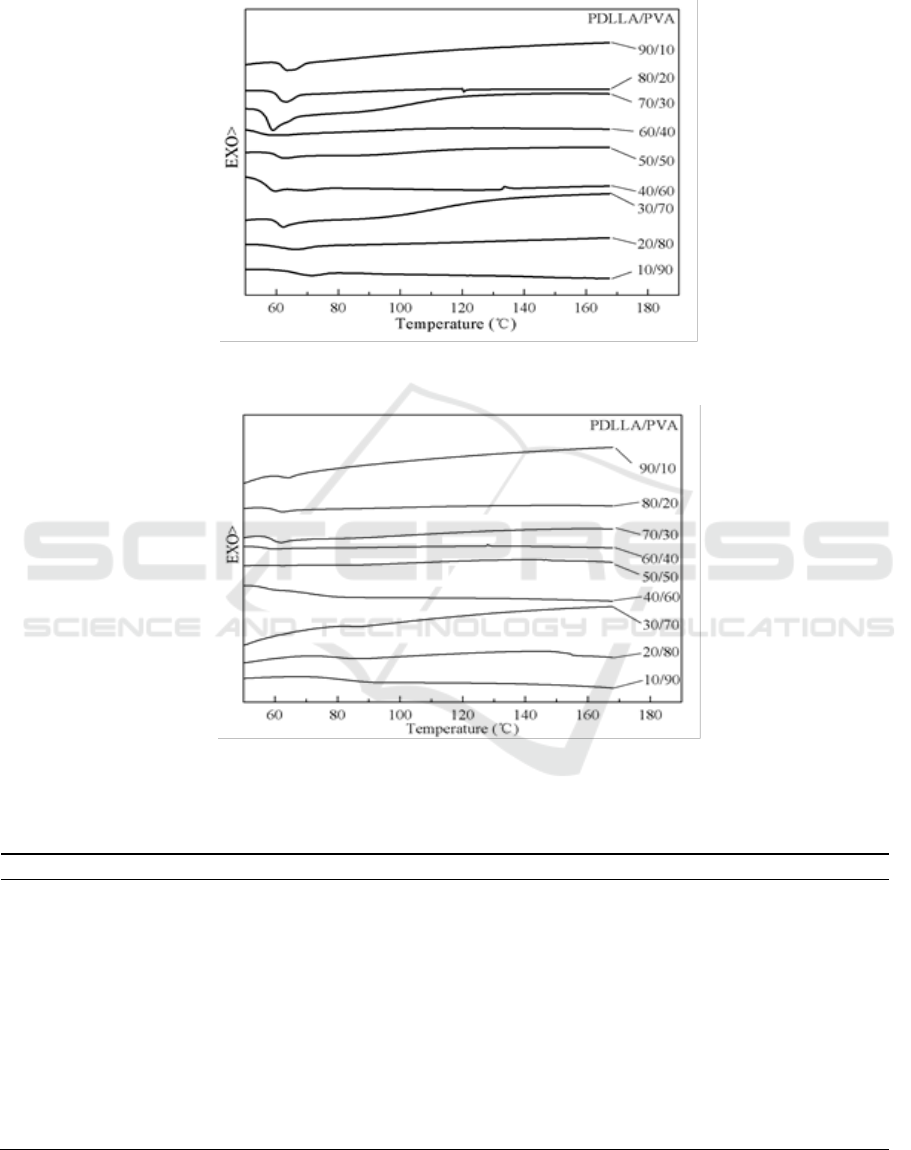
Shuai et al. [33] studied the miscibility of PLLA/PVA blends, finding immiscibility or partially
miscible because DSC measurements detected two series of isolated and rather composition-
independent T
g
. They used hexafluoro isopropanol as solvents, and blend solutions were cast onto
Teflon dishes after polymers were codissolved. The solvent was allowed to evaporate at room
temperature for 2 weeks, and films were dried in vacuo for 3 weeks at 60°C . But in this progress,
DMF was used as PDLLA/PVA mixed solvents, and polymers were codissolved at 140°C . Figures 3
and 4 were the first and second scans of PDLLA/PVA blends, respectively. All results of
PDLLA/PVA blends showed similar traces, it can be seen from Figure 3 and Figure 4 that there was
only one T
g
(also see Table 1.) in each blend. The glass transition temperature of the blends rose with
the increasing of PVA which all temperatures were higher than those of neat PDLLA (59°C ). We can
also see that PDLLA and PVA were miscible, and the compatibility in the second scan was better
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
418
than that in the first one, especially when the component of PDLLA was low. Nevertheless, FTIR
results (see the following section) were much more conclusive than DSC ones, supporting nearly
pure phases.
Figure 3. First scan DSC traces of PDLLA/PVA blends.
Figure 4. Second scan DSC traces of PDLLA/PVA blends.
Table 1. DSC results for PDLLA/PVA blends with different composition.
PDLLA/PVA (wt%)
T
g
-first (°C )
T
g
-second (°C )
100/0
59
59
90/10
62
61
80/20
61
60
70/30
58
59
60/40
-
59
50/50
59
71
40/60
57
-
30/70
61
81
20/80
10/90
63
67
80
79
0/100
85
85
Effects of Hydrogen Bond Interaction on the Miscibility of Poly (D, L-lactide) Composites Materials
419
PEG
600
was used to plasticized poly (L-lactide) by Kulinski et al. [34]. It indicated that T
g
was
decreased with increasing of plasticizers. The DSC data for the amorphous PLA also showed clearly
a decrease of T
g
due to enhanced of segmental mobility of PLA chains caused by the presence of
plasticizer, increasing with the PEG
300
in this study. As can be seen from Figure 5, at low mixed
temperature (40°C ), the first and second heating scan showed good compatibility for
PDLLA/PEG300 blends. No significant differences were found during two heating scans. The results
suggested that as-casting PDLLA/PEG
300
blends were miscible in nearly pure polymer.
Figure 5. First and second scan DSC traces of PDLLA/PEG
300
blends.
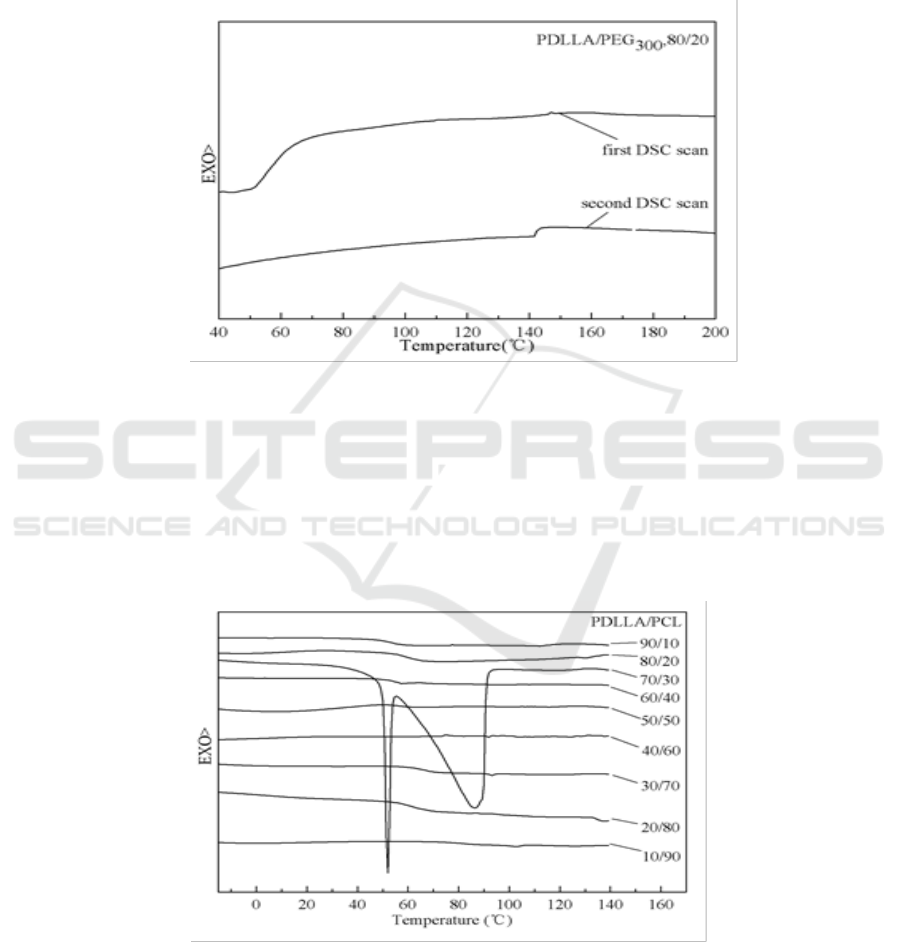
Many scholars have already proved that PLA and PCL are immiscible or partially miscible [17,
35-38]. Figure 6 and Figure 7 showed that the first scan was consistent with the second one. PDLLA
was immiscible with PCL when the mixed temperature was low. Even after the blends were heated,
the results were still unsatisfactory. However, Solid-state NMR studies indicated the presence of
phase separation. In addition, it may be not remove all residual crystals during first heating which the
glass transition of PDLLA and melting of PCL were overlapped in some DSC thermograms showing
in Figure 6.
Figure 6. First scan DSC traces of PDLLA/PCL blends.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
420
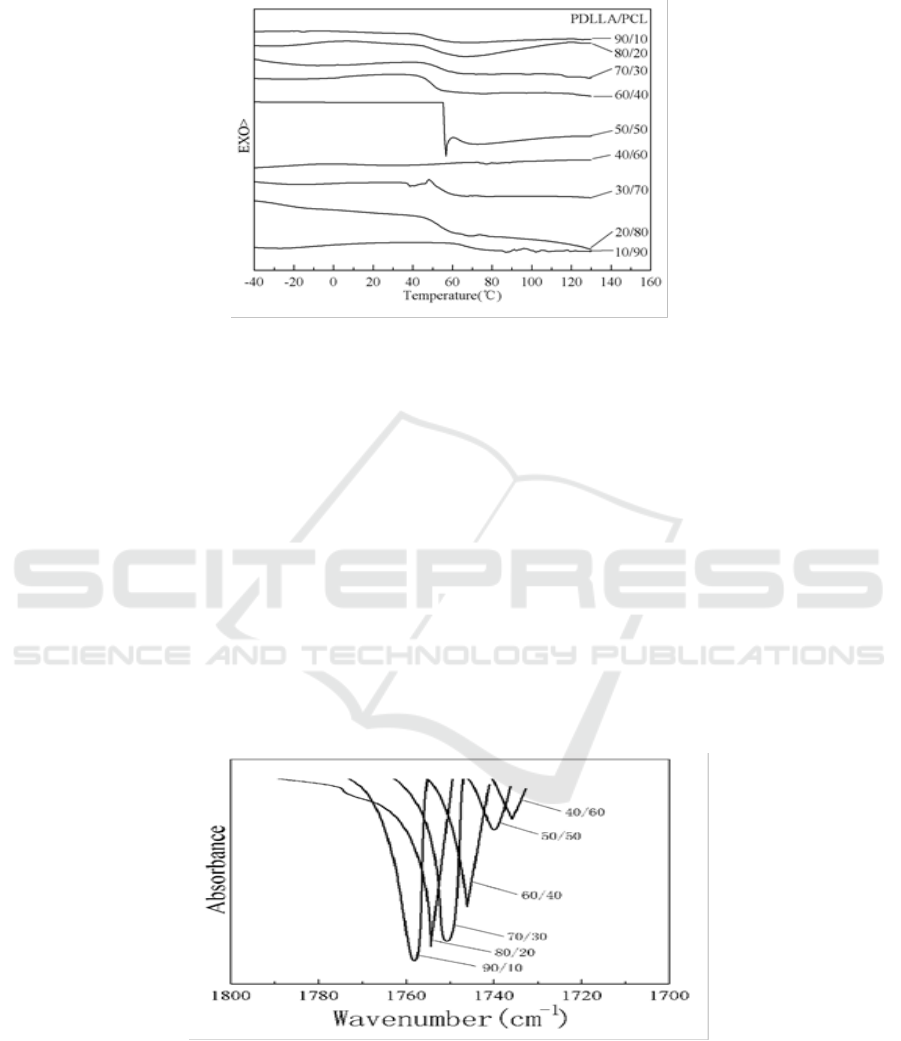
Figure 7. Second scan DSC traces of PDLLA/PCL blends.
3.2. FT-IR results
FT-IR were widely used to study hydrogen bonding in blends of polymers [39]. From the chemical
structures in Figure 1 we can see that PDLLA is easier to form hydrogen bonding with PVA and PEG
than PCL. Shuai et al. also proved that hydrogen bonding appeared between PLLA and PVA by FT-
IR and Solid-state NMR.
Infrared spectroscopy has been widely used to investigate specific interactions in polymer blends
in which the driving force for miscibility was hydrogen bonding. All these studied blends have been
analyzed, and hydrogen bonding were confirmed during PDLLA/PVA and PDLLA/PEG
300
blends.
Figure 8 and Figure 9 were IR spectra of PDLLA/PVA blends after the second DSC scan. It can be
seen from the results that as PVA is increased, hydroxyl group does more and more effect on
carboxyl band (C=O). Figure 8 shows the peak of carboxyl become smaller and smaller. Figure 9
shows that the hydrogen bond (O-H…O=C) makes the peak of hydroxyl band (O-H) become wider
and shift to red. However, for the blends of PDLLA and PCL, both of the two IR peaks are almost
unchanged, indicating that no hydrogen bond is formed between them.
Figure 8. FTIR of PDLLA/PVA blends from 1700-1800 cm
-1
.
Effects of Hydrogen Bond Interaction on the Miscibility of Poly (D, L-lactide) Composites Materials
421
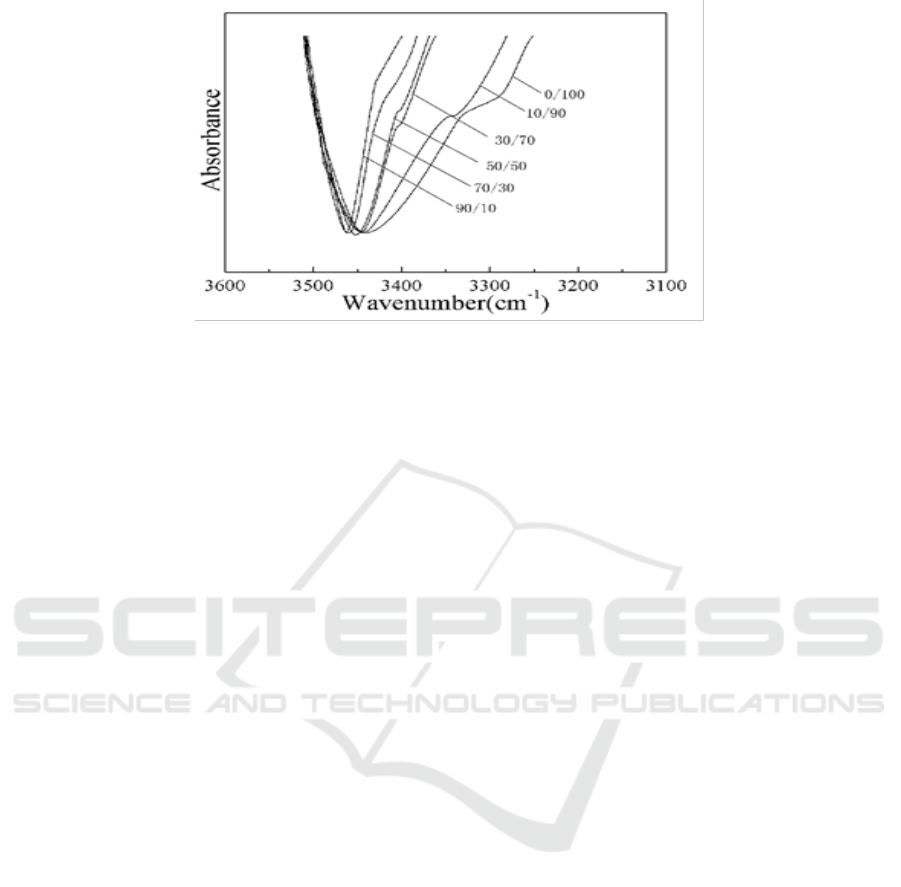
Figure 9. FTIR of PDLLA/PVA blends from 3100-3600 cm
-1
.
3.3. Blend miscibility
According to DSC data and FTIR results, miscibility of PDLLA/PVA, PDLLA/PEG
300
and
PDLLA/PCL were studied. The solitary T
g
of PDLLA/PVA and PDLLA/PEG
300
blends indicated
good miscibility. The chemical structures of the blending polymers support the DSC results also.
There were hydroxyl groups at PVA main chain and PEG end group. Miscibility should become
better due to hydrogen bonding (O-H…O=C) which produced from hydroxyl groups and carboxyl
groups. Hence, miscibility would be weakened because there was no hydrogen bonding during
PDLLA and PCL. During heating, thermal motion increased both chain mobility and the probability
of hydrogen bonding contacts. Rigidity of mail chain would affect miscibility for blends, and second
heating results of PDLLA/PVA blends showed better miscibility than first scans also. Lesser
molecular weight of PEG would increase miscibility of PDLLA/PEG
300
, and also enhance
plasticizing agent role.
4. Conclusions
DSC results show that PDLLA/PVA and PDLLA/PEG
300
were miscible in whole composition range
and PDLLA/PCL blends were immiscible. Miscibility of PDLLA/PVA after twice heating scan was
better than as-casting blends because thermal motion increased both chain mobility and the
probability of hydrogen bonding contacts. With increasing of PEG
300
its plasticizing agent role were
obvious in blends of PDLLA/PEG
300
due to lesser molecular weight. According to FT-IR analysis,
results of DSC were confirmed. There were hydrogen bonds in blends of PDLLA/PVA and
PDLLA/PEG
300
, but there were no hydrogen bonds in PDLLA/PCL. Hence, miscibility of
PDLLA/PVA and PDLLA/PEG
300
were enhanced by hydrogen bonding. As a result, the effect of
hydrogen bonding and the mixed temperature are very important in the miscibility of blends.
References
[1] Wanda S, Piotr D, Michal S, Joanna R, Marta M and Marek K 2008 Degradation Study of
Polymers from Renewable Resources and their Compositions in Industrial Composting Pile
Macromol. Symp. 272 pp 132-135
[2] Murali M R, Singaravelu V, Manjusri M, Sujata K B and Amar K M 2013 Biobased plastics
and bionanocomposites: Current status and future opportunities Prog. Polym. Sci. 38 pp
1653–89
[3] Richard A G and Bhanu K 2016 Biodegradable polymers for the environment Science 297 pp
803-807
[4] Zhu Y Q, Romain C and Williams C K 2016 Sustainable polymers from renewable resources
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
422

Nature 540 pp 354–362
[5] Zeng J B, Li K A and Du A K 2015 Compatibilization strategies in poly(lactic acid)-based
blends RSC Adv. 5 pp 32546-65
[6] Joanna R, Wanda S, Mariya K and Darinka C 2015 Polyester-based (bio)degradable polymers
as environmentally friendly materials for sustainable development Int. J. Mol. Sci. 16 pp
564–596
[7] Astrid L, Guillermo M, Betânia H L and AndréL J, Rubens M F 2012 Poly-lactic acid
synthesis for application in biomedical devices - A review Biotechnol. Adv 30 pp 321-328
[8] Mauck S C,Wang S, Ding W, Rohde B J, Fortune C K, Yang G, Ahn S K and Robertson M L
2016 Biorenewable tough blends of polylactide and acrylated epoxidized soybean oil
compatibilized by a polylactide star polymer Macromolecules 49 pp 1605–15
[9] BagóJ R, Pegna G J, Okolie O, Mohiti-Asli M, Loboa E G and Hingtgen S D 2016 Electrospun
nanofibrous scaffolds increase the efficacy of stem cell-mediated therapy of surgically
resected glioblastoma Biomaterials 90 pp 116–125
[10] Cifuentes S C, Gavilán R, Lieblich M, Benavente R and González-Carrasco J L 2016 In vitro
degradation of biodegradable polylactic acid/magnesium composites: Relevance of Mg
particle shape Acta Biomater. 32 pp 348–357
[11] Tsuji H 2016 Poly (lactic acid) stereocomplexes: A decade of progress Adv. Drug Deliv. Rev.
107 pp 97–135
[12] Saeidlou S, Huneault M A, Li H and Park C B 2012 Poly (lactic acid) crystallization, Prog.
Polym. Sci. 37 pp 1657-77
[13] Peng L C, Chai Y, Liu Y and Zhang P Y 2008 Progress in fully biodegradable polylactice
blends China Plastics. 22 pp 1-8
[14] Spinella S, Cai J, Samuel C, Zhu J, McCallum S A, Habibi Y, Raquez J M, Dubois P and
Gross R A 2015 Polylactide/Poly (ω-hydroxytetradecanoic acid) reactive blending: A green
renewable approach to improving polylactide properties Biomacromolecules 16 pp 1818–
26
[15] Xu H, Yang X, Xie L and Hakkarainen M 2016 Conformational footprint in hydrolysis-
induced nanofibrillation and crystallization of poly (lactic acid) Biomacromolecules 17 pp
985–995
[16] Yao Q, Cosme J L, Xu T, Miszuk J M, Picciani P S, Fong H and Sun H 2017 Three
dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved
stem cells osteogenic differentiation and cranial bone formation Biomaterials 115 pp 115–
127
[17] Han L, Xie Q, Bao J, Shan G, Bao Y and Pan P 2017 Click chemistry synthesis, stereocomplex
formation, and enhanced thermal properties of well-defined poly (L-lactic acid)-b-poly (D-
lactic acid) stereo diblock copolymers Polym Chem. 8 pp 1006–16
[18] Zhang K, Jr C S, Washburn N R, Antonucci J M, Lin-Gibson S 2005 In situ formation of
blends by photopolymerization of poly (ethylene glycol) dimethacrylate and polylactide
Biomacromolecules 6 pp 1615-22
[19] Guo J, Qiao J and Zhang X 2016 Effect of an alkalized-modified halloysite on PLA
crystallization, morphology, mechanical, and thermal properties of PLA/halloysite
nanocomposites J. Appl. Polym. Sci. 133 44272
[20] Zhao X, Luo J, Fang C and Xiong J 2015 Investigation of polylactide/poly(ε-
caprolactone)/multi-walled carbon nanotubes electrospun nanofibers with surface texture
RSC Adv. 5 pp 99179–87
[21] McDonald P F, Geever L M, Lyons J G and Higginbotham C L 2010 In vitro degradation and
drug release from polymer blends based on poly(DL-lactide), poly(L-lactide-glycolide) and
poly(ε-caprolactone) J. Mater. Sci. 45 pp 1284-92
Effects of Hydrogen Bond Interaction on the Miscibility of Poly (D, L-lactide) Composites Materials
423

[22] Zhang L, Goh S and Lee S 1998 Miscibility and phase behavior of poly (D, L-lactide)/poly-(p-
vinylphenol) blends J. Appl. Polym. Sci. 70 pp 811-816
[23] Blümm E and Owen A 1995 Miscibility, crystallization and melting of poly(3-hydro
xybutyrate)/ poly(L-lactide) blends Polymer 36 pp 4077-81
[24] Schneider E M, Taniguchi S, Kobayashi Y, Hess S C, Balgis R, Ogi T, Okuyama K and Stark
W J 2017 Efficient Recycling of Poly (lactic acid) Nanoparticle Templates for the Synthesis
of Hollow Silica Spheres ACS Sustain Chem. Eng. 5 pp 4941–47
[25] Williams C K and Hillmyer M A 2008 Polymers from Renewable Resources: A Perspective
for a Special Issue of Polymer Reviews Polym. Rev. 48 pp 1–10
[26] Li Z B, Tan B H, Lin T T and He C B 2016 Recent advances in stereocomplexation of
enantiomeric PLA-based copolymers and applications Prog. Polym. Sci. 62 pp 22-72
[27] Pretula J, Slomkowski S and Penczek S 2016 Polylactides-Methods of synthesis and
characterization Adv. Drug Deliv. Rev. 107 pp 3-16
[28] Wang T, Wang S, Luo R, Zhu C, Akiyama T and Zhang Z 2016 Microencapsulation of phase
change materials with binary cores and calcium carbonate shell for thermal energy storage
Appl. Energy 171 pp 113–119
[29] Elin R and Heike B 2017 Drug release studies from lipid nanoparticles in physiological media
by a new DSC method J. Control. Release 256 pp 91-100
[30] Puentes J, Restrepo-Zapata N C, Chaloupka A, Duddleston L L, Rudolph N and Osswald T A
2017 Quasi-isothermal DSC testing of epoxy adhesives using initial fast heating rates J.
Appl. Polym. Sci.134 pp 45425
[31] Sar A 2016 Thermal energy storage characteristics of bentonite-based composite PCMs with
enhanced thermal conductivity as novel thermal storage building materials Energy Convers.
Manag. 117 pp 132–141
[32] Dong Y and Feng S S 2006 Nanoparticles of poly (D, L-lactide)/methoxy poly(ethylene
glycol)-poly(D,L-lactide) blends for controlled release of paclitaxel J. Biomed. Mater. Res.
A. 78 pp 12-19
[33] Shuai X, He Y, Asakawa N and Inoue Y 2001 Miscibility and phase structure of binary blends
of poly(L-lactide) and poly (vinyl alcohol) J. Appl. Polym. Sci. 81 pp 762-772
[34] Kulinski Z and Piorkowska E 2005 Crystallization, structure and properties of plasticized
poly(L-lactide) Polymer 46 pp 10290-00
[35] Xiong Z, Liu F, Gao A L, Lin H B, Yu X M, Wang Y Z and Wang Y 2016 Investigation of the
heat resistance, wettability and hemocompatibility of a polylactide membrane via surface
crosslinking induced crystallization RSC Adv. 6 PP 20492–99
[36] Lv Q L, Wu D F, Xie H, Peng S, Chen Y and Xu C J 2016 Crystallization of poly(ε-
caprolactone) in its immiscible blend with polylactide: insight into the role of annealing
histories RSC Adv. 6 pp 37721-30
[37] Urquijo J, Guerrica-Echevarría G and Eguiazábal J I 2015 Melt processed PLA/PCL blends:
Effect of processing method on phase structure, morphology, and mechanical properties J.
Appl. Polym. Sci. 132 pp 42641
[38] Jing Z, Shi X, Zhang G and Gu J 2017 Synthesis and properties of poly(lactide)/poly(ε-
caprolactone) multiblock supramolecular polymers bonded by the self-complementary
quadruple hydrogen bonding Polymer 121 pp 124–136
[39] Chen C C, Chueh J Y, How T, Huang H M and Lee S Y 2003 Preparation and characterization
of biodegradable PLA polymeric blends Biomaterials 24 pp 1167–73
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
424
