
Effect of LiH on the Dehydriding Property of ɑ-AlH
3
Composite
C W Duan
1,*
, J Zhang
2
, Y L Zhang
1
and J L Ma
3
1
Department of Environmental Science and Engineering, North China Electric
Power University, Baoding, 071003, China
2
Department of Computed Tomography and Magnetic Resonance Imaging, Baoding
First Center Hospital/First Aid Center, Baoding, 071003, China
3
School of Information Science and Engineering, Hebei University of Science and
Technology, Shijiazhuang, 050018, China
Corresponding author and e-mail: C W Duan, Duancw@ncepu.edu.cn
Abstract. As a promising hydrogen storage composite, the ɑ-AlH
3
/LiCl nano-composite was
prepared by mechanochemical synthesis method. However, so far there is no investigation on
the dehydriding property of this composite. In present work, the hydrogen desorption
property of this composite is investigated systematically. When heating temperature goes
from 80 to 140 °C, the isothermal desorption measurements show that 9.93 wt% of hydrogen
is released from the composite and the kinetic of the dehydrogenation improved with the
temperature raised. Moreover, thermal analysis by differential scanning calorimeter (DSC) is
used to research the de-hydriding process of the ɑ-AlH
3
/LiCl nano-composite, from which the
composite composes one step with the direct decomposition of the α phase. With the LiH
introduced into the AlH
3
/LiCl nano-composite, the dehydrogenation measurements reveal that
the de-hydriding kinetics of this system was also improved. According to the DSC results, it
is found that the LiH addition can reduce the activate energy of the de-hydriding reaction.
1. Introduction
In order to seek a new and green energy source, hydrogen is regarded to be a perfect carrier for
energy storage, transportation application and the application of hydrogen-fueled cells due to its
unique feature of non-toxicity, high energy density and promising performance in fuel cells [1]. In
recent years, on-board hydrogen storage posed considerable technical challenges that could be
detrimental to the application of fuel cells [2]. Therefore, a lightweight, effective and high capacity
hydrogen storage material should be developed for hydrogen storage [3]. Among the various light
hydrides, AH
3
(alane) with a higher gravimetric hydrogen capacity exceeding 10 wt%, a lower
desorption temperature (100-200 °C) and a minor dehydriding enthalpy, is acknowledged as a
fascinating material, and attracted more and more attentions for its potential as a hydrogen storage
candidate [4].
It is well known that the non-solvated AlH
3
with seven variations in its crystal structures, such as
phase, were firstly synthesized by the direct reaction of LiAlH
4
and AlCl
3
in
diethyl ether solvent [5]. Based on the thermodynamic calculation, it is deduced that the and other
538
Duan, C., Zhang, J., Zhang, Y. and Ma, J.
Effect of LiH on the Dehydriding Property of -AlH3 Composite.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 538-544
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

phase of AlH
3
can spontaneously decompose at 298 K [6]. But, it was verified by Graetz that the
decomposition of AlH
3
polymorphs is still not feasible at room temperature mainly due to the effect
of dehydriding kinetics [7].
Among the seven AlH
3
polymorphs, the unit cell, lattice parameters and
bridge bonds of the α phase were determined by Turley et. al. in 1969, from which only one type of
corner connected AlH
6
octahedra consist in this structure [8]. In view of the above structural analysis,
α-AlH
3
is more stable than other polymorphs. According to the formation enthalpy and formation
entropy of α phase calculation, the Gibbs formation energy for α phase at 300 K can be obtained with
a value of 31 KJ/mol H
2
[9]. Therefore, it can be concluded from the above thermal analysis that α-
AlH
3
is recognized to be more stable than other phase.
It was also verified by Liu that the initial dehydriding temperature of the fresh γ-AlH
3
is still to be
about 130 °C [2], which probably hinders the application of AlH
3
as an hydrogen storage material. A
promising approach proposed by Gutowska that the nano-sized metal hydride could increase the
dehydriding kinetics without doping of catalyst [10]. Thus, the searching group focused on the
improving desorption kinetics with a ball milling method [11]. Orimo et. al. Also found that only
milling the as-prepared AlH
3
could reduce the dehydriding temperature and accelerate the desorption
rates [9].
But, their works were mainly focus on the thermodynamics of milled AlH
3
, not the
dehydriding reaction kinetics. Similarly, Graetz reported that an fresh nano-sized AlH
3
synthesized
by wet chemical method also exhibits an desirable decomposition temperature less than 100 °C [12],
and have a high H
2
yield which can approach the theoretical hydrogen content of AlH
3
(10 wt.%)
without needing furthermore ball milling or mixing as-prepared AlH
3
with small levels of the alkali
metal hydrides [7]. Nevertheless, the above mentioned methods are not perfect due to complexity and
extremely sensitive during the process of synthesizing nano-sized AlH
3
. Namely, an additional
approach such as ball milling was performed to product nano-sized hydride from the obtained AlH
3
.
Furthermore, the desolvating process for removing large quantities of organic solvents from the
solvates is uneconomical and hazardous.
Recently, the mechanochemical method is considered to be both green and economical powerful
tool to obtain metal hydrides [13]. This mechano-chemical method was later investigated by research
group that employed a desirable way to synthesize nano-sized AlH
3
[14]. Nevertheless, as a
promising hydrogen storage media, the dehydriding property of as-milled AlH
3
composite was still
not investigated and discussed systematically. More effort should be focus on the dehydriding
kinetics of -AlH
3
nano-composite. In our previous work, the -AlH
3
/LiCl nano-composite was
successfully prepared by a liquid state reaction between LiH and AlCl
3
[15]. In present study, the de-
hydriding process and de-hydriding kinetics of this nano-composite is thoroughly investigated.
2. Experimental
The ɑ-AlH
3
/LiCl nano-composite was firstly prepared by liquid state reaction milling with LiH,
AlCl
3
and ionic liquid [15]. To investigate the effect of LiH on the ɑ-AlH
3
dehydriding property, the
excessive LiH (5 mol %) was added into reaction system directly. The mixed powder was put in a
ball-milling canister. Ball milling was performed via a planetary-type QM-SP4 device attached to
500 cm
3
ball-milling canisters. During the mechanochemical reaction, hydrogen pressure in the vial
was kept above 5 MPa. The as-milled composite was eventually formed after ball milling.
Isothermal and Non-isothermal dehydriding tests were carried out on a home-made special
vacuum apparatus made reactor. The ɑ-AlH
3
/LiCl nano-composite was loaded into a stainless holder.
In order to investigate dehydriding kinetics of composite systemically, the as-milled samples were
powdered at different temperatures of 80, 120 and 140°C . The time required for the full dehydriding
reaction was fixed at 5,000 s, respectively. During the temperature programmed desorption (TPD)
process, the rate of heating temperature as well as the vacuity in closed special equipment were
controlled by a computer and monitored in situ with digital vacuum gauges. To investigate the
dehydriding process of the ɑ-AlH
3
nano-composite, the TPD measurements were performed from 40
Effect of LiH on the Dehydriding Property of -AlH3 Composite
539
to 240 °C with a heating rate of 3°C /min. The hydrogen content desorbed from the composite was
calculated in terms of the vial vacuum change. Based on the stoichiometric weight of AlH
3
calculated
by the chemical reaction, the dehydriding curves of as-milled AlH
3
/LiCl and LiH/AlH
3
/LiCl nano-
composite could be obtained. Thermal analysis was studied by differential scanning calorimetry
(DSC) on a DSC METTLER TOLEDO instruments. In order to prevent the sample form oxidizing,
the samples was sealed into a crucible (constructed from Al) in glove box and quickly transferred to
the instrument in T-zero pans. During the measurement, the argon was flowed at 20 mL/min to
minimize the sample exposure to air. Subsequently, the samples were heated from 40 to 240°C at
various rates of 3, 5, 10, 15°C /min, respectively. It is noted that the as-milled products which mixed
with ionic liquid were filtrated and dried in a vacuum before de-hydriding measurement.
3. Results and Discussion
3.1. Non-isothermal dehydriding analysis
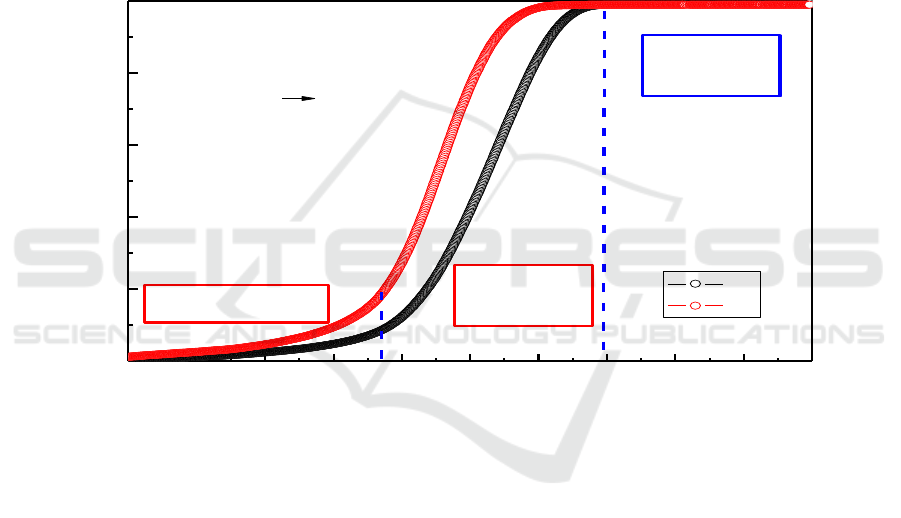
Figure 1. TPD curves of (I) as-milled ɑ-AlH
3
/LiCl nano-composite, (II) ɑ-AlH
3
/LiCl nano-composite
doped with LiH.
The non-isothermal desorptions of the ɑ-AlH
3
/LiCl nano-composite and the same product with LiH
addition are shown in Figure 1. The curves of non-isothermal desorptions are investigated by the
temperature-programmed-desorption (TPD) measurements from 40 to 240°C with a heating rate of
3°C/min. It is shown from Figure 1 that the obtained nano-composite starts to release hydrogen
approximately to be 60°C and subsequently follows a slow desorption process between 60 and 115°C.
The hydrogen content released from the composite is just 0.86 wt % after the temperature is
gradually increased to 115°C. This hydrogen capacity is much lower than the theoretical gravimetric
hydrogen capacity of pure AlH
3
, suggesting that a small proportion of decomposition occurred with a
relatively slow heating rate. When the heating temperature is gradually increased to 180°C, the
desorption process slows down with a maximum hydrogen desorption capacity of 9.93 wt% for the
as-milled nano-composite, which is approximately to be the theoretical hydrogen capacity with a
value of 10.1 wt%. It can be concluded that the all the AlH
3
in this composite decomposed into Al
under a higher de-hydriding temperature. Overview the TPD curves of the composites in Figure 1, it
is indicated that the hydrogen desorption of the ɑ-AlH
3
/LiCl nano-composite exhibits a three-stage
process. Namely, the introduction stage starts at 60°C and ends at 115°C, and subsequently an
40 60 80 100 120 140 160 180 200 220 240
0
2
4
6
8
10
acceleratory
period
Al+LiCl+H
2
-AlH
3
/LiCl
stage3:
stage1 and stage2:
stage3
stage2
Temperature(C)
Hydrogen desorption(wt%)
(I)
(II)
stage1
induction period
decay period
reaction completed
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
540
accelerated period of decomposed process as well as an final stage identified as the decay period.
However, it is observed from the curve of (II) in Figure 1 that the de-hydriding rate of ɑ-AlH
3
/LiCl
composite was faster than the product without LiH addition. Furthermore, AlH
3
doped with LiH
presents a more desirable de-hydriding kinetics. This is attributed to the LiH has some effects on the
decomposition kinetics of AlH
3
. These results have good correspondence with the our previous work
in which Zn and Zr can accelerate the de-hydriding reaction of AlH
3
[16, 17]. Thus, it can be deduced
that LiH also can act as an impediment to overgrowth of metal Al and subsequently improve the
dehydriding kinetics during the desorption process.
3.2. Isothermal dehydriding property of the ɑ-AlH
3
/LiCl nano-composite
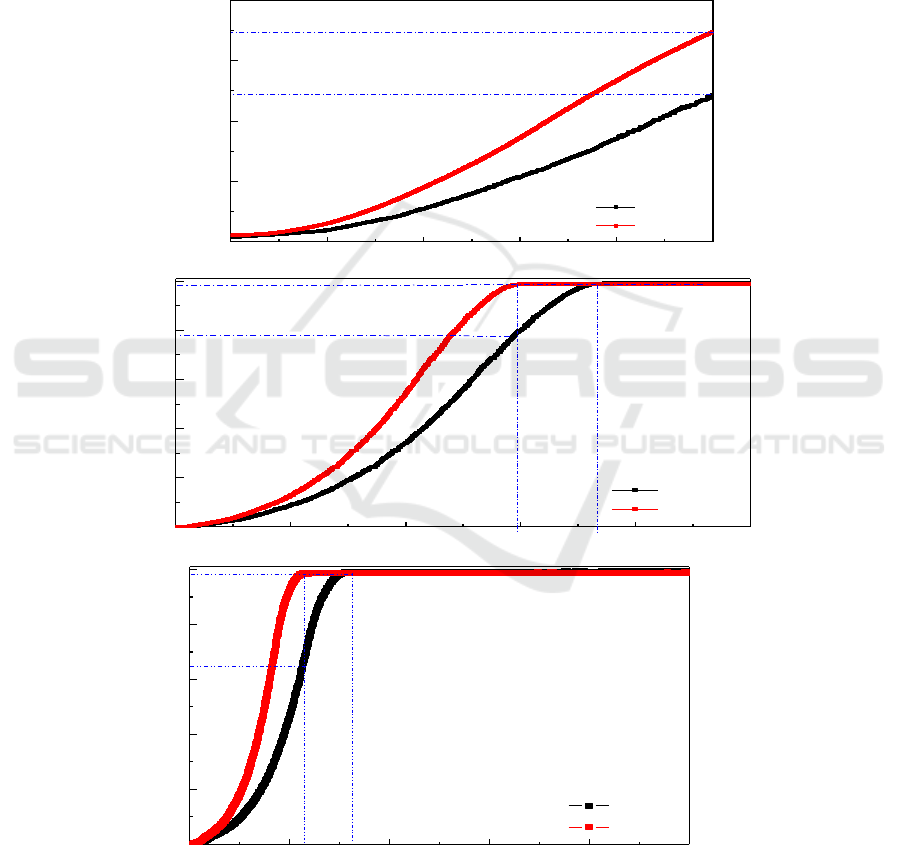
Figure 2. The de-hydriding kinetics curves of (I)α-AlH
3
/LiCl nano-composite and (II) the same
composite added with LiH at various temperatures: (a) 80°C, (b) 120°C, (c) 140°C.
0 1,000 2,000 3,000 4,000 5,000
0
2
4
6
8
4.84
(I)
(II)
6.95
Hydrogen desorption(wt%)
Desorption time(s)
(a)
0 1,000 2,000 3,000 4,000 5,000
0
2
4
6
8
10
7.82
9.89
9.93
(I)
(II)
Hydrogen desorption(wt%)
Desorption time(s)
(b)
0 1,000 2,000 3,000 4,000 5,000
0
2
4
6
8
10
Hydrogen desorption(wt%)
9.89
6.48
9.93
(I)
(II)
Desorption time(s)
(c)
Effect of LiH on the Dehydriding Property of -AlH3 Composite
541

To explore the effect of LiH on the synthesis of the α-AlH
3
/LiCl composite, Figure 2 shows the
curves of dehydriding kinetics of the as-milled α-AlH
3
composite at various temperatures and
different time intervals. As can be seen in Figure 2 that the rate of dehydrogenation accelerated as the
temperature rose to 140°C. Additionally, from the curves for dehydrogenation reaction, it can be
conjectured that LiH has remarkable effect on the dehydrogenation reaction of the α-AlH
3
/LiCl nano-
composite. When the de-hydriding temperature is fixed at 80°C for 5,000 s, it can be seen from the
Figure 2(a) that the hydrogen desorption content of as-milled product reached merely 4.84 and 6.95
wt% respectively, indicating that the dehydriding reaction was still not complete under this condition.
Compared with the AlH
3
/LiCl composite without adding LiH, AlH
3
has a significant advantage on
dehydriding property with the same hydrogen content at 80 °C for 3,736 s. Although the dehydriding
curve exhibited an undesirable property, the as-milled product still have an advantage in de-hydriding
properties compared with the much lower hydrogen content of 1.9 wt% derived from the as-milled
AlH
3
which fully decomposed from room temperature to 200°C [14]. Furthermore, the value
described above, was higher than the 0.48 wt% hydrogen content of crude α-AlH
3
measured by
Graetz. et. al.[3, 6, 7]. By increasing the reaction temperature to 120°C for 3,000 s, the hydrogen
content of α-AlH
3
/LiH composite increased rapidly to 9.89 wt%, suggesting that almost all the AlH
3
decomposed, much more than at lower temperature. Even dopped with LiH, it was demonstrated by
Sandrock that only 4 wt % H
2
can be obtained from the AlH
3
/LiH composite in the the first four
hours [18]. Furthermore, the same hydrogen content could be obtained by heating the reaction
mixture at 140°C for the 1,140s, which implied that the dehydrogenation rate was clearly accelerated
by increasing the temperature. It is obvious that the AlH
3
added with LiH has a more desirable
dehydriding dynamics. Consequently, the LiH probably come into play with the decomposition
kinetics of α-AlH
3
and can accelerate the de-hydriding reaction of α-AlH
3
. Although fresh
synthesized nanoscale α-AlH
3
has an advanced dehydrogenation property, it was reported by Graetz
that fully decomposed time at 138°C can be achieved even within 1,800s [12]. Therefore, it can be
concluded that the α-AlH
3
nano-composite doped with LiH exhibits an excellent advantage in de-
hydriding property.
3.3. The de-hydriding kinetics of the ɑ-AlH
3
/LiCl nano-composite
In order to gain an deep insight into the de-hydriding process of the α-AlH
3
/LiCl/LiH nano-
composite, further supporting evidence can be obtained from the DSC curves in Figure 3. Figure 3(a)
shows DSC curves of as-milled α-AlH
3
composite added with LiH at several heating rates. It is
obvious that the desorption curves of the composite added with LiH still have a similar peaks with
that of un-doped composite. The whole DSC curves contain only one endothermic peak at a elevated
temperature of 40-240 °C. This endothermic peak derives from α-AlH
3
decomposition is consistent
with Liu report [2]. It was found that the endothermic peak between 80 and 190 °C is assigned to the
de-hydriding reaction of the α phase [2]. This indicates that no new phase was formed in the product.
Namely, α-AlH
3
can not react with LiH during the de-hydriding process. Based on the above non-
isothermal analysis, the corresponding de-hydriding temperature of α-AlH
3
is remarkably reduced
with the LiH added into the composite. Thus, it can be deduced that the LiH have some effects on the
decomposition kinetics of α-AlH
3.
To determinate the value of apparent activation energies (Ea) for
this dehydriding process, the desorption kinetics of the α-AlH
3
/LiCl nano-composite was studied by
using the Kissinger’s method. Moreover, the relationship among the activation energy (Ea), the
heating rate (c), and the peak temperature of de-hydriding (T
P
) in the DSC curve can be formulated
by following Kissinger’s equation:
Ln (c/T
2
p
) = - (E
a
/RT
p
) + A (1)
Figure 3(b) shows the activation energy of the de-hydriding reaction based on parameters
obtained from DSC measurements. The apparent activation energy for the hydrogen desorption of α-
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
542
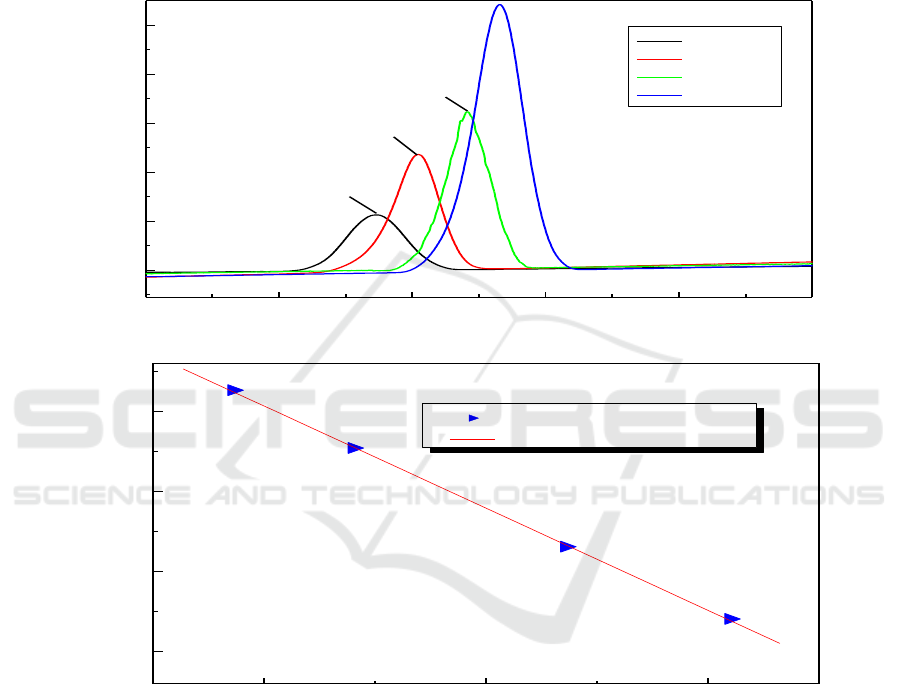
AlH
3
in the composite were estimated to be 52.9 KJ/mol, which are slightly lower than that of the as-
milled AlH
3
/LiCl nano-composite without LiH addition (56.8 KJ/mol) [15]. This value is also lower
than result reported by Gabis who found that the apparent activation energy of the dehydrogenation
of α-AlH
3
is 104 KJ/mol [19]. This decrease in the kinetic barrier is contribute to the remarkable
improvement in the hydrogen desorption kinetics, and the decrease in the activation energy can
describe the above TPD and DSC results vigorously, from which the α-AlH
3
/LiCl nano-composite
added with LiH has a more desirable de-hydriding kinetics.
Figure 3. (a) DSC curves of the α-AlH
3
nano-composite doped with LiH in temperature ranges from
40 to 240°C , (b) The apparent energy for the decomposition obtained from DSC measurements.
4. Conclusions
The α-AlH
3
/LiCl nano-composite which was prepared by mechanochemical methods releases about
9.9 wt% of hydrogen in the temperature of 40-240°C. Combining the DSC analysis, the de-hydriding
process of the α-AlH
3
nano-composite was found, that is, the obtained α-AlH
3
dehydride directly
during the dehydriding process. Moreover, the α-AlH
3
nano-composite doped with LiH exhibits an
excellent advantage in de-hydriding property. With the LiH added into composite, the activation
energy of de-hydriding of α-AlH
3
was reduced from 56.8 to 52.9 kJ/mol. Thus, it can be deduced that
LiH can remarkably improve the de-hydriding kinetics of the α-AlH
3
nano-composite.
40 80 120 160 200 240
0.0
0.1
0.2
0.3
0.4
0.5
146.1C
136.8C
121.2C
109.5C
3C/min
5C/min
10C/min
15C/min
DSC, heat flow (w/g)
Temperature(C)
(a)
0.0024 0.0025 0.0026
-11.0
-10.5
-10.0
-9.5
Experimental points
Fitting curve
K=-6371
Ea=52.9KJ/mol
ln(c/Tp
2
)
1/Tp
(b)
Effect of LiH on the Dehydriding Property of -AlH3 Composite
543

Acknowledgments
The present work was supported financially by the Natural Science Foundation of Hebei Province
(Grant E2018502054) and the Fundamental Research Funds for the Central Universities (Grant
2017MS141) This work was also supported by the National Major Science and Technology Program
for Water Pollution Control and Treatment (Grant 2017ZX07101-001-007).
References
[1] Contestabile M, Offer G J, Slade R, Jaeger F and Thoennes M 2011 Energ. Environ. Sci. 4
3754-72.
[2] Liu H, Wang X, Dong Z, Cao G, Liu Y, Chen L and Yan M 2013 Int. J. Hydrogen Energy 38
10851-56.
[3] Graetz J 2009 Chem. Soc. Rev. 38 73-82.
[4] Banach E M, Stil H A and Geerlings H 2012 J. Mater. Chem. 22 324-7.
[5] Brower F M, Matzek N E, Reigler P F, Rinn H W, Roberts C B, Schmidt D L and Terada K
1976 J. Am. Chem. Soc. 98 2450-3.
[6] Graetz J and Reilly J J 2006 J. Alloys. Compd. 424 262-5.
[7] Graetz J and Reilly J J 2005 J. Phy. Chem. B 109 22181-5.
[8] Turley J W and Rinn H W 1969 Inorg. Chem. 8 18-22.
[9] Orimo S, Nakamori Y, Kato T, Brown C and Jensen C M 2006 Appl. Phys. A 83 5-8.
[10] Gutowska A, L Li, Shin Y, C M Wang, X S Li, Linehan J C, Smith R S, Kay B D, Schmid B,
Shaw W, Gutowski M and Autrey T 2005 Angew. Chem. Int. Ed. 44 3578-82.
[11] Huot J, Pelletier J F, Lurio L B, Sutton M and Schulz R 2003 J. Alloys Compd. 348 319-24.
[12] Graetz J, Reilly J J, Kulleck J G and Bowman R C 2007 J. Alloys. Compd. 446 271-5.
[13] Fernandez J A, Aguey-Zinsou F, Elsaesser M, Ma X Z, Dornheim M, Klassen T and Bormann
R 2007 Int. J. Hydrogen Energy 32 1033-40.
[14] Gupta S, Kobayashi T, Hlova I Z, Goldston J F, Pruski M and Pecharsky V K 2014 Green
Chem. 16 4378-88.
[15] C W Duan, L X Hu and J L Ma 2018 J. Mater. Chem. A 6 6309-18.
[16] C W Duan, L X Hu and Y Sun 2015 RSC Adv. 5 17104-8.
[17] C W Duan, L X Hu Y Sun and Z P Wan 2016 RSC Adv. 6 74215-24.
[18] Sandrock G, Reilly J and Graetz J 2005 Appl. Phys. A 80 687-90.
[19] Gabis I, Dobrotvorskiy M and Evard E 2011 J. Alloy. Compd. 509 S671-4.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
544
