
Attapulgite-based Adsorbent for Mercury (II) Removal in
Aqueous Solution
M Li
1, *
, B Li
1
, Y Wang
1
, R J Wang
1
and Q D Zeng
2,3
1
Department of Environmental Science & Engineering, North China Electric Power
University, Baoding, China
2
National Center for International Research on Green Optoelectronics, South China
Normal University, Guangzhou, China
3
Guangdong Provincial Key Laboratory of Optical Information Materials and
Technology and Institute of Electronic Paper Displays, South China Academy of
Advanced Optoelectronics, South China Normal University, Guangzhou, China
Corresponding author and e-mail: M Li, ming2999@126.com
Abstract. A new attapulgite-based adsorbent P-ATP was successfully prepared to remove
mercury (II) from aqueous solutions via grafting reactions. The structures of the products
were characterized by Fourier Transform Infrared Spectra (FTIR) and scanning electron
microscope (SEM) analysis. The adsorption process was investigated by the batch adsorption
experiments. The influence factors including pH, temperature, and contact time were all
discussed. To further understand the adsorption process between adsorbents and the heavy
metals, pseudo-second-order kinetic model was introduced, and Langmuir adsorption
isotherm model was also used to describe the adsorption process. The adsorption capacity of
Hg (II) on P-ATP was calculated to be as high as 181.16 mg/g. Furthermore, the adsorbent
could be reused after 7 times of adsorption-desorption cycles.
1. Introduction
In recent years, heavy metal pollution in water environments becomes much more serious than ever
before [1, 2]. Kinds of heavy metal ions are discharged into rivers, lakes, oceans, and underground
waters with the rapid development of economy and modern industry. Among the released heavy
metals, mercury is considered to be one of the most toxic elements due to its great harm to the human
body. Furthermore, mercury could be enriched in the creatures by the food chain, thus, it makes the
creatures at the top of the food chain suffer more damage [3]. Moreover, excess mercury may cause
dysfunction of liver, kidney, brain tissue, and other serious problems [4].
Up to now, a great number of chemical and physical techniques have been used to eliminate
mercury in water, such as chemical precipitation, ion-exchange, membrane filtration, electrochemical
treatment. However, the above methods have obvious disadvantages: either operational complexity
or high cost [5]. Therefore, adsorption technique becomes a useful and effective approach for the
elimination of mercury in water [6, 7]. Attapulgite (ATP) is a kind of hydrated magnesium aluminum
silicate mineral, which is very cheap and easy to be chemically modified via reactions with hydroxyl
558
Li, M., Li, B., Wang, Y., Wang, R. and Zeng, Q.
Attapulgite-based Adsorbent for Mercury (Ii) Removal in Aqueous Solution.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 558-564
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

groups on the surface or in the porous structures. Besides, attapulgite is not soluble in water, making
it easy to be separated from aqueous solutions.
In this work, we report a new attapulgite-based adsorbent for the removal of mercury (II) in
aqueous solution. 3-aminopropyltriethoxysilane (KH550) was employed as the coupling reagent, and
pyromellitic dianhydride (PMDA) was introduced as the chelating groups for metal ions. The
adsorption capacity of mercury (II) was calculated to be as high as 181.16 mg/g. Pseudo-second-
order model and Langmuir adsorption isotherm model were also investigated to further understand
the adsorption process.
2. Experimental section
2.1. Materials Synthesis
2.1.1. Pretreatment for attapulgite. First, the crude attapulgite was grind to 400 meshes with sieves,
and divided into 30 g per group for the following acid pickling. The attapulgite was added in
hydrochloric acid (4 mol/L) with a mass ratio of 1:10. The above mixture was heated for 4 hours at
90
o
C, then filtrated and washed by deionized water for several times until the filtrate is neutral. The
filtration residue was dried and ready for modification. The pretreatment for attapulgite is to release
the hydroxyl groups to active the activity of the raw material.
2.1.2. Modification of attapulgite. 60 g acid-activated attapulgite was added into 1L deionized water,
and stirred in a flask to be dispersed uniformly. 30 mL 3-aminopropyltriethoxysilane (KH550) was
then added into the above mixture and stirred for half an hour. After the reaction, the mixture was
filtrated, washed by deionized water and dried in an oven.
2.1.3. Functionalization of attapulgite. 0.932g pyromellitic dianhydride (PMDA) was dissolved in 60
mL DMF (N, N-dimethylformamide), then 466 mg 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (EDC) and 372.8 mg N-hydroxysulfosuccinimide sodium salt (NHS) were added into
the above mixture. The solution was stirred for 30 minutes, and 2 g KH550-grafted attapulgite was
added afterwards. The mixture was stirred for 1 more hour at 80
o
C. When the reaction was finished,
the mixture was filtrated and the filtration residue was dried to obtain the adsorbent P-ATP.
2.2. Characterizations of the products
The functional groups of ATP and its derivatives were identified on a Fourier Transform Infrared
Spectra (FTIR, Nicolet 6700, USA) in the range of 400-4000 cm
-1
. The morphology of the products
and elements analysis was operated by a JEOL-7800 scanning electron microscope (SEM, Japan).
The concentration of the mercury (II) in solution was tested using an atomic fluorescence
spectrometer AFS-8220 (Titan instruments, China), and the concentrations of the other heavy metal
ions (including Cu (II), Pb (II), and Cr (VI)) in solution were measured by an Atomic adsorption
spectrometer (Agilent Technologies 200 Series AA, USA).
3. Results and discussion
3.1. Characterizations of the adsorbents
FTIR was used to identify the functional groups in the adsorbent, and it could also indicate the
reaction process via the characteristic peaks in different reaction steps. As is shown in Figure 1., the
adsorption peaks at 1029 and 471 cm
-1
are attributed to the Si-O-Si bonds, and the peak at 800 cm
-1
is
attributed to the stretching vibration of Al-O-Si bonds [8].The peak at 882 cm
-1
is attributed to the
Fe-O-Fe bonds. In addition, the peak at 1641 cm
-1
indicates the zeolitic water [9]. The band at 1441
cm
-1
is attributed to the carbonate minerals in ATP [10]. After the pretreatment of the raw material,
Attapulgite-based Adsorbent for Mercury (Ii) Removal in Aqueous Solution
559
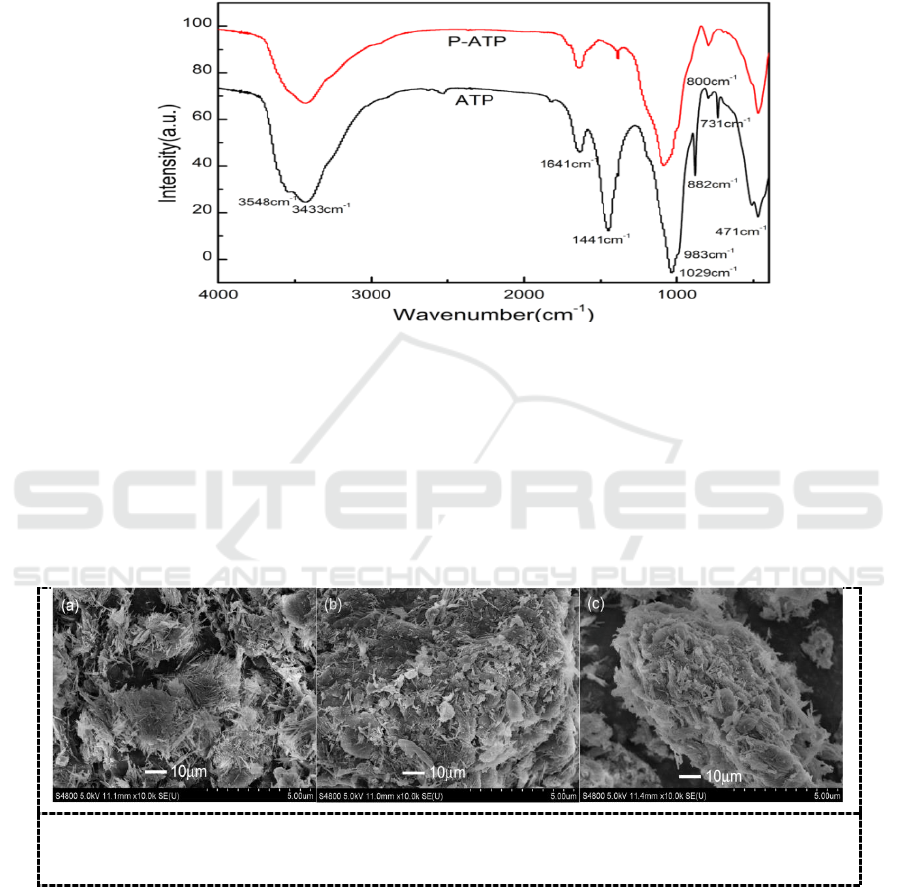
the carbonate was decomposed by the acid, thus, the adsorption peak disappeared in the FTIR spectra
of modified ATP. Furthermore, the adsorption peak at 1640 cm
-1
is assigned to N-H deformation
vibration, which is similar to the adsorption peak of zeolitic water, therefore, it is difficult to identify
the above two peaks in FTIR spectra.
Figure 1. FTIR spectra of ATP and P-ATP.
The SEM images of ATP and its derivatives are shown in Figure 2 and 3. The raw ATP shows
blocks of close packed fibres, and these fibres reunites together like needles. The difference of
surface morphology between ATP and KH550-grafed ATP are not so obvious, and only the
mesopores between the blocks turn smaller. It is worth noting that remarkable morphologic changes
has taken place on the PDMA modified ATP (named as P-ATP) that the surface of P-ATP becomes
much rougher than its precursor, lots of tiny needles spread out on the surface of the block of the
basic material.
Figure 2. (a) SEM images of ATP (a), KH550-grafted ATP (b), and PMDA modified
ATP (P-ATP) (c).
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
560
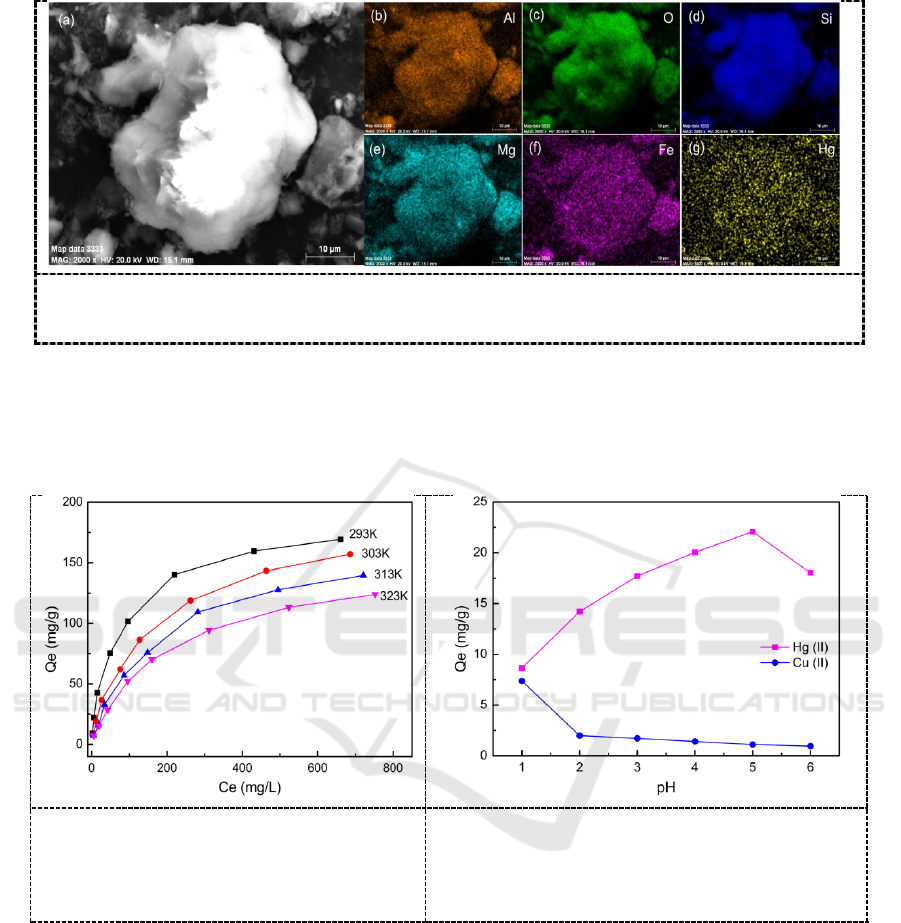
Figure 3. (a) SEM image of Hg-loaded P-ATP and elemental distribution images of Al
(b), O (c), Si (d), Mg (e), Fe (f), and Hg (g).
3.2. Effect of temperature, pH, and contact time on the adsorption
To evaluate the efficiency of the as-prepared adsorbent, the adsorption process for heavy metals
should be conducted under the optimum adsorption conditions, including the operation temperature,
pH, and contact time.
Figure 4. Effect of different temperatures
operated on Hg (II) removal process.
(pH=5.0, contact time=2 h, adsorbent
dose= 1 g/L).
Figure 5. Effect of pH on Hg (II) and Cu (II)
removal in aqueous solutions. (T=293 K,
contact time= 2 h, adsorbent dose= 1 g/L).
The effect of temperature on the adsorption is shown in Figure 4, lower temperature is confirmed
to be better for Hg (II) removal. Besides, the influence of pH is also investigated from pH=1.0 to
pH=6.0 as show in figure 5. Higher pH is not considered due to the precipitation generated when the
concentration of Hg (II) goes higher. By contrast, lower pH is not suitable for the test either, because
H
+
is also positively charged and much smaller than mercury ions, making it a relatively competitive
opponent to occupy the finite active sites on the P-ATP particles. Thus, pH=5.0 is selected for the
following experiments.
Attapulgite-based Adsorbent for Mercury (Ii) Removal in Aqueous Solution
561
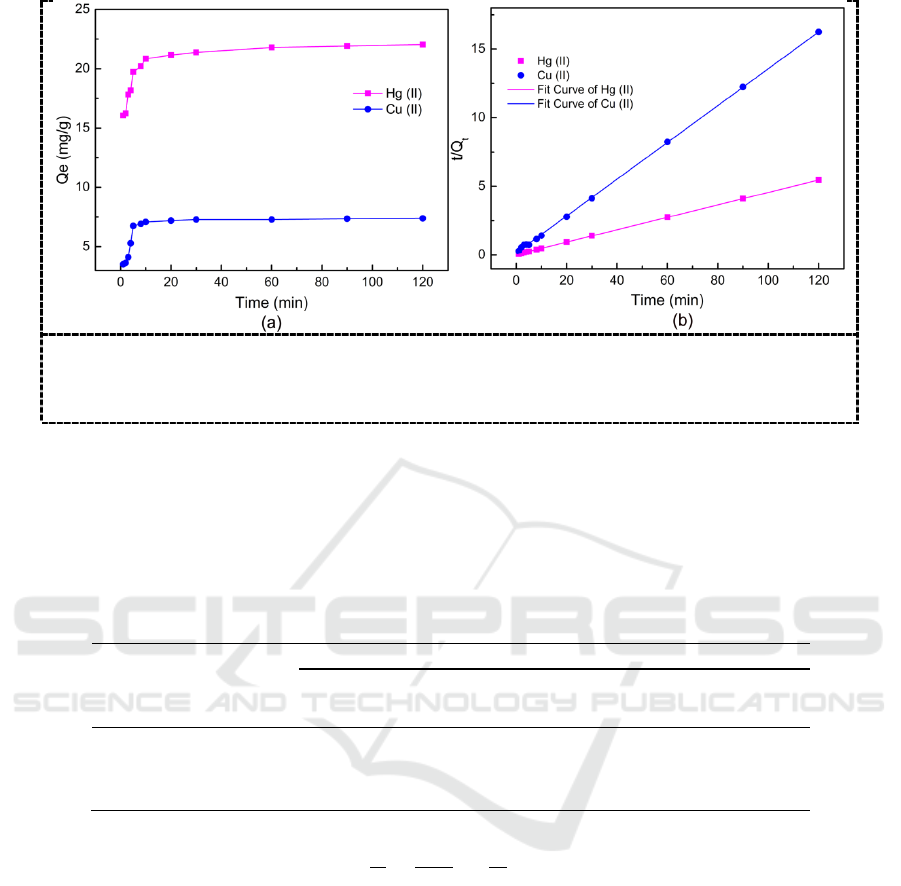
Figure 6. (a) Effect of contact time for Hg (II) and Cu (II) removal; (b) Pseudo-second-
order sorption kinetics of Hg (II) and Cu (II) (pH=5.0, initial metal ion concentration= 50
mg/L, T=293 K, adsorbent dose= 1 g/L )
Contact time is also an important factor when the adsorbent is used for the practical application.
Figure 6 illustrates the influence of contact time for Hg (II) and Cu (II) removal. At the initial stage
in the first 30 minutes, the adsorption rate is very fast due to the unsaturation of the adsorbent, as the
adsorption sites are saturated, the adsorption equilibrium is achieved and the adsorption rate is
decreased.
Table 1. Kinetic parameters for adsorption of Hg (II) and Cu (II).
Metal ions
Q
e,exp.
(mg·g
-1
)
Pseudo-second-order model
Q
e,cal.
(mg·g
-1
)
k
2
×10
2
(g·mg
-1
·min
-1
)
R
2
Hg (II)
22.03
22.22
4.35
0.9999
Cu (II)
7.38
7.46
10.5
0.9997
(1)
The pseudo-second-order model is introduced to describe the adsorption kinetic process which
could be expressed as the formula (1), where t is the contact time, Qt and Qe are the adsorption
capacity at time t and adsorption equilibrium, respectively. k
2
is the rate constant of pseudo-second-
order kinetic model. The relevant kinetic data are calculated and listed in Table 1. The experimental
Qe data is close to the calculated ones in this model with the correlation coefficient values R
2
very
close to 1, which could be attributed to a chemical adsorption on the P-ATP.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
562
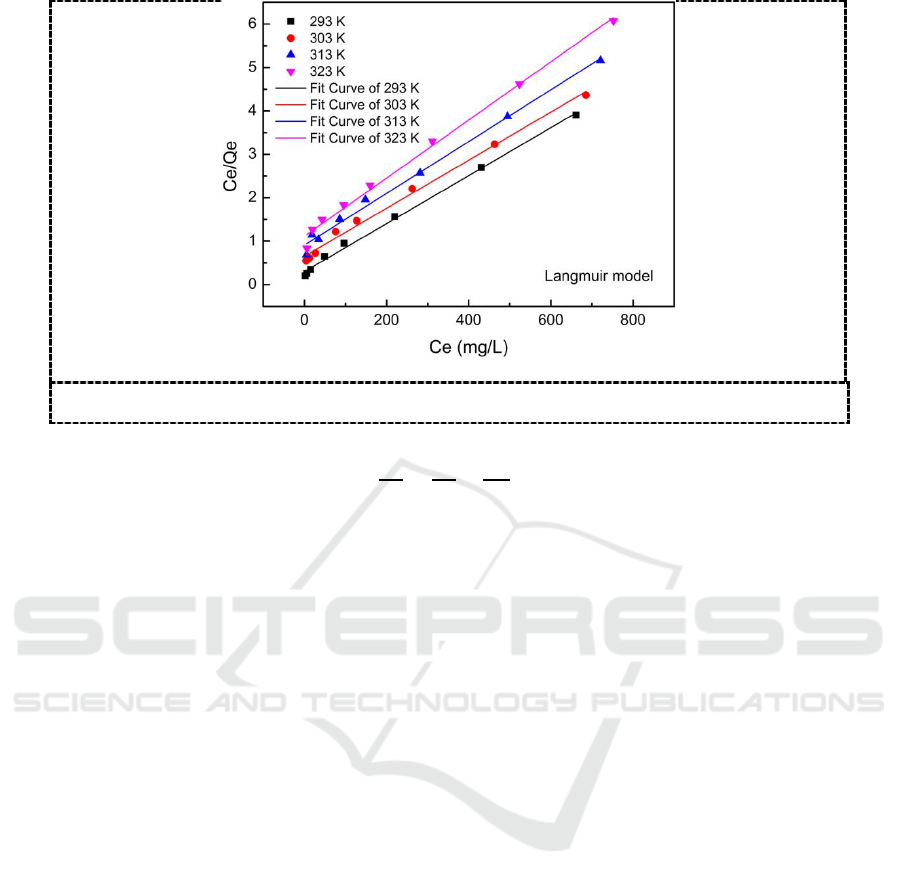
Figure 7. Langmuir adsorption model for Hg (II) removal on P-ATP.
(2)
In order to analyse the adsorption properties of P-ATP, Langmuir adsorption isotherm model is
used to describe the adsorption process. The Langmuir adsorption isotherm model is described as
formula (2) [11], where Ce is the equilibrium concentration, Qe is the amount of Hg (II) adsorbed on
P-ATP at equilibrium. Qm and b are the maximum adsorption capacity and Langmuir constant,
respectively. The fit curves of the Langmuir adsorption isotherm model at different temperatures are
shown in Figure 7. The Langmuir model well fits the experimental data with all R
2
close to 1, and we
could calculate the equilibrium adsorption capacity of Hg (II) on P-ATP as high as 181.16 mg/g with
R
2
= 0.9983. Moreover, the results indicate a monolayer adsorption process on P-ATP [12].
3.3. Regeneration of the adsorbent
The adsorbent was regenerated with 1 M HNO
3
solution. After seven times of adsorption-desorption
cycles, the adsorption capacity for Hg (II) were measured to be 106.4 mg/g, exhibiting good
regeneration properties as well.
4. Conclusions
In this work, PMDA was successfully grafted on ATP with KH550 as a coupling linker and PMDA
as a chelating group. The obtained adsorbent exhibited enhanced removal efficiency towards mercury
(II) from aqueous solutions. The experimental data could be described with pseudo-second-order
kinetic model which indicated a chemical adsorption on P-ATP. Moreover, the adsorption process
could be well fitted with Langmuir adsorption isotherm model and could be attributed as a monolayer
adsorption. Meanwhile, the adsorption capacity was calculated to be 181.16 mg/g, and the adsorbent
exhibited good regeneration properties as well.
Acknowledgement
This work was supported financially by the Fundamental Research Funds for the Central Universities
(Grant nos. 2016MS111 and 2016MS110).
References
[1] Zhou G, Luo J, Liu C, Chu L, and Crittenden J 2018 Water Res. 131 246
Attapulgite-based Adsorbent for Mercury (Ii) Removal in Aqueous Solution
563

[2] Fakhre N and Ibrahim B 2018 J. Hazard. Mater. 343 324
[3] Kobielska P, Howarth A, Farha O, and Nayak S 2018 Coordin. Chem. Rev. 358 92
[4] Behjati M, Baghdadi M, and Karbassi A 2018 J. Environ. Manage. 213 66
[5] Maruyama T, Terashima Y, Takeda S, Okazaki F, and Goto M 2014 Process Biochem. 49 850
[6] Mohamed E, Amr A, Marwa T, Maher M, and Tarek M 2015 J. Environ. Sci. Heal. A 50 1072
[7] Zhu Z, Yang X, He L, and Li W 2012 RSC Adv. 2 1088
[8] Lu Z, Hao Z, Wang J, and Chen L 2016 J Ind. Eng. Chem. 34 374
[9] Shen Z, Gao W, Li P, Wang X, Zheng Q, Wu H, Ma Y, Guan W, Wu S, Yu Y, and Ding K
2016 Talanta 159 194
[10] Rusmin R, Sarkar B, Biswas B, Churchman J, Liu Y, and Naidu R 2016 Appl. Clay Sci. 95 134
[11] Kumari S and Chauhan G 2014 ACS Appl. Mater. Interfaces 6 5908
[12] Cao J, Wang C, Fang F, and Lin J 2016 Environ. Pollut. 219 924
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
564
