
Preparation of Flexible Epoxy Resin Containing
Polyurethane Segments
C Z Liu
1,2
, M M Sun
1,2
, J X Jia
3
, G Xue
1
, C Y Song
1,2
, J H Li
1
, X G Zhang
1,2
, L
Wang
1
, M Zhao
1
and B Zhang
1,2,*
1
Institute of Petrochemistry, Heilongjiang Academy of Sciences, Harbin,
Heilongjiang 150040, P. R. China.
2
Institute of Advanced Technology, Heilongjiang Academy of Sciences, Harbin,
Heilongjiang 150020, P. R. China.
3
AVIC huiyang aviation propeller co., Ltd, Baoding, Hebei 071000, P. R. China.
Corresponding author and e-mail: B Zhang, zhangbin_hipc@126.com
Abstract. Epoxy resin is applicable for adhesives, coatings, electronic packaging, composite
materials and other fields. In this paper, an original flexible epoxy resin (FEP) was
synthesized by introducing polyurethane segments into the main chain. The formation of FEP
was characterized using FTIR and
1
H MNR methods. Subsequently, the bonding properties of
the FEP/bisphenol A diglycidyl ether (DGEBA) blends were studied, and 4,4’-
diaminodiphenylmethane (DDM) was used as a curing agent. The results showed that the
introduction of FEP made a great improvement for T-peel strength, while the lap shear
strength at different temperature increased firstly and then decreased with the increasing of
FEP content.
1. Introduction
Epoxy resin has been widely used in many industrial applications, such as adhesives, coatings and
composite materials for many years [1-2]. Similar to other thermosetting resins, the cured epoxy resin
shows a brittle behavior due to high crosslink density. The commonly approach used to improve the
toughness of epoxy resins is the incorporation of second phase particles, such as rubber elastomer,
thermoplastic polymer, inorganic nanoparticle, liquid crystalline polymer, and hyperbranched resin
reported in many papers [2-5].
In recent years, polyurethane (PU) has attracted much research attention due to its versatile
applications. Generally, PU was composed of polyether or polyester “soft” segments and a varieties
of isocyanate (-NCO)-based “hard” segments [6]. It was found that the modified epoxy resin with PU
elastomer showed a good toughness and low temperature performance because of the formation of an
interpenetrating network structure. The urethane groups (-NHCOO-) in the PU molecular chain also
play a positive impact on mechanical strength, impact resistance and bonding strength of epoxy resin.
However, the traditional PU is sensitive to moisture for the existence of free -NCO groups, and the
corresponding modified epoxy adhesive exhibits a poor storage stability [7]. Therefore, in this work,
Liu, C., Sun, M., Jia, J., Xue, G., Song, C., Li, J., Zhang, X., Wang, L., Zhao, M. and Zhang, B.
Preparation of Flexible Epoxy Resin Containing Polyurethane Segments.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 565-570
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
565

a polyurethane prepolymer capped with glycidyl was synthesized and used to toughen epoxy resin.
On one hand, it showed good compatibility with epoxy resin as the existence of co-crosslinking
reaction. On the other hand, it was expected that the toughness of epoxy resin could be significantly
improved by the flexible polyether chain of PU.
2. Experimental Methods
2.1. The synthesis of hydroxymethyl phenyl glycidyl ether (MPEP)
The raw materials, 4-hydroxybenzyl alcohol (MP, 0.1 mol), epichlorohydrin (ECH, 0.2 mol) and
potassium carbonate (0.12 mol) were placed into a three-necked flask equipped with a mechanical
stirrer, reflux condenser and thermometer, and butanone (80 mL) was added as a solvent. The
reaction mixture was stirred at 80°C for 40 h, and the excess ECH and solvent were then removed by
vacuum distillation. A yellowish transparent liquid was obtained. The synthesis route of MPEP is
shown in Figure 1(a).
2.2. The synthesis of polyurethane prepolymer
Polyether polyol (M
2
, Mn=2000) was degassed and dried in a round flask under high vacuum at
110°C for 3 h. After the temperature was cooled to 80°C , a certain amount of toluene-2,4-
diisocyanate (TDI) (molar ratio of TDI and M
2
is 2.0) was then added dropwise, and the reaction was
continued for another 3 h at 80°C protected by dry nitrogen gas. The reaction process was monitored
by FTIR technique to obtain a polyurethane prepolymer. The reaction route is shown in Figure 1(b).
2.3. Preparation of flexible epoxy resin containing polyurethane segments (FEP)
one equivalent of as-prepared polyurethane prepolymer and two equivalents of MPEP were added
into a reaction kettle equipped with a nitrogen inlet. The mixture was continuously mixed at 80°C
until the -NCO groups disappeared. The synthesis route of FEP is shown in Figure 1(c).
2.4. Preparation of DGEBA/FEP blends
The FEP/DGEBA ratios of 0%, 20% ,40%, 60% and 80% by mass of DGEBA were used. The resin
blends containing stoichiometric amount of DDM curing agent were polymerized gradually at 80°C
for 1 h, 120°C for 2 h and 150 °C for another 2 h in an oven.
2.5. Characterization and measurements
The structure of the as-prepared monomer was verified by
1
H NMR (Bruker AVANCE-500 nuclear
magnetic resonance spectrometer) with a proton frequency of 400 MHz. The solvent was deuterated
chloroform and tetramethylsilane (TMS) was used as an internal standard. Fourier transform infrared
(FTIR) spectra were recorded by a Bruker-VECTOR22. The samples were brushed on a potassium
bromide window and the FTIR spectra were obtained at a resolution of 4 cm
-1
at room temperature.
The lap shear strength (LSS) test specimens were prepared according to GB/T 7124-2008. The
aluminium alloy plates (2024-T3) were used as adherend in this study, which were polished with a 80
grade emery paper and then cleaned with ethyl acetate prior to bonding. The T-peel strength (T-PS)
were tested by an electro-mechanical testing machine (Instron 5969) at room temperature. The test
speed is 100 mm/min.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
566
(a)
(b)
(c)
Figure 1. Preparation process of flexible epoxy resin containing polyurethane segments: (a) MPEP,
(b) polyurethane prepolymer and (c) FEP.
3. Results and discussion
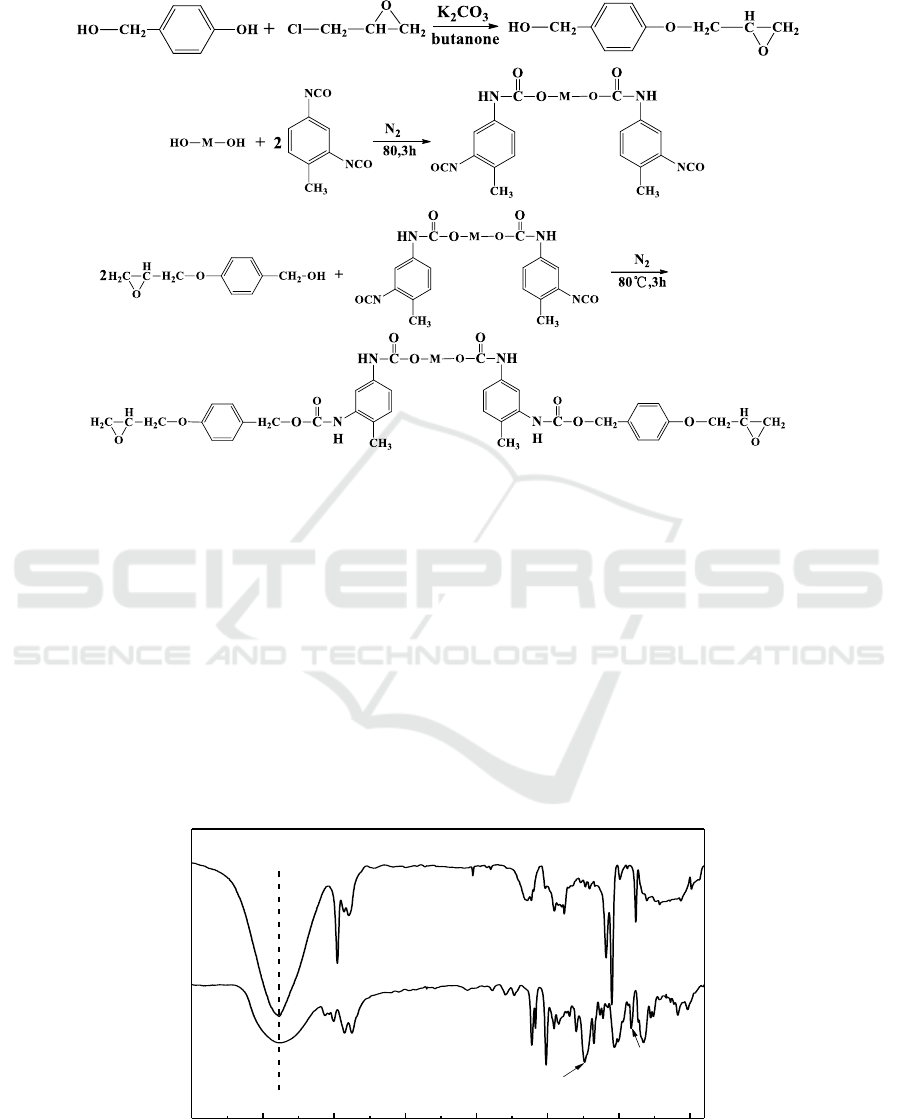
The FT-IR spectra of MP and MPEP are shown in Figure 2. It can be seen that MPEP exhibited the
characteristic absorption at 1237 cm
-1
, which was attributed to the C-O-C stretching vibration. The
characteristic absorption of epoxy ring appeared at 915 cm
-1
. Moreover, the absorption at
approximately 2863 and 2924 cm
-1
related to the asymmetric and symmetric stretching vibrations of
methylene (-CH
2
-), were also founded. As shown in Figure 3, the chemical structures of MP and
MPEP were also characterized by
1
H NMR. The peak observed at 9.2 ppm for MP was assigned to
the phenolic hydroxyl group (Ph-OH) structure, which has been disappeared for MPEP. The
resonance signals of epoxide protons observed at 3.7 ppm were assigned to the methylene f, and the
peaks at 2.5 and 2.9 ppm were attributed to the methylene g [8]. These results demonstrated that
MPEP have the expected chemical structures.
4000 3500 3000 2500 2000 1500 1000 500
1237
915
Transmittance (%)
Wave number (cm
-1
)
3450
MP
MPEP
Figure 2. FTIR spectra of MP and MPEP.
Preparation of Flexible Epoxy Resin Containing Polyurethane Segments
567
10 8 6 4 2 0
Chemical shift (ppm)
MPEP
MP
e
b
a
DMSO
H
2
O
c,d
g
H
2
O
DMSO
f
e
b
a
c,d
Figure 3.
1
H NMR spectra of MP and MPEP.
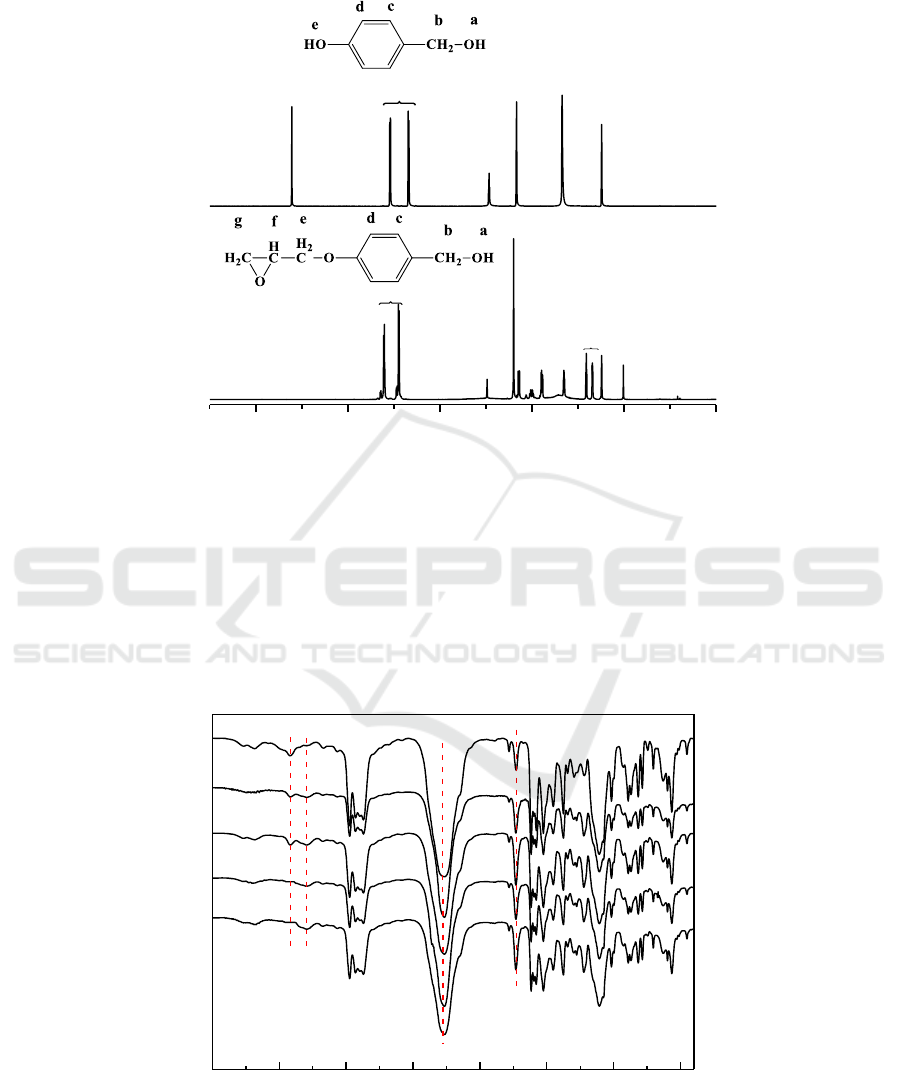
The NCO-terminated polyurethane prepolymers were synthesized by M
2
and TDI. The synthetic
process was continuously monitored by a FTIR spectrometer. As shown in Figure 4 the decay for the
intensity of -NCO (2267 cm
-1
) and Ph-OH (3464 cm
-1
) absorption peaks can be used to monitor the
conversion of functional groups during reaction. Meanwhile, the peak at 3298 cm
-1
(N-H) increased
observably as the reaction time prolonged. After polymerized at 80°C for 3 h, the Ph-OH
characteristic peak at 3464 cm
-1
has disappeared.
4000 3500 3000 2500 2000 1500 1000 500
Transmittrance(%)
Wave number (cm
-1
)
0h
1h
2h
3h
3.5h
2267
1729
3464
3298
Figure 4.FTIR spectra of polyurethane prepolymers at different time.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
568
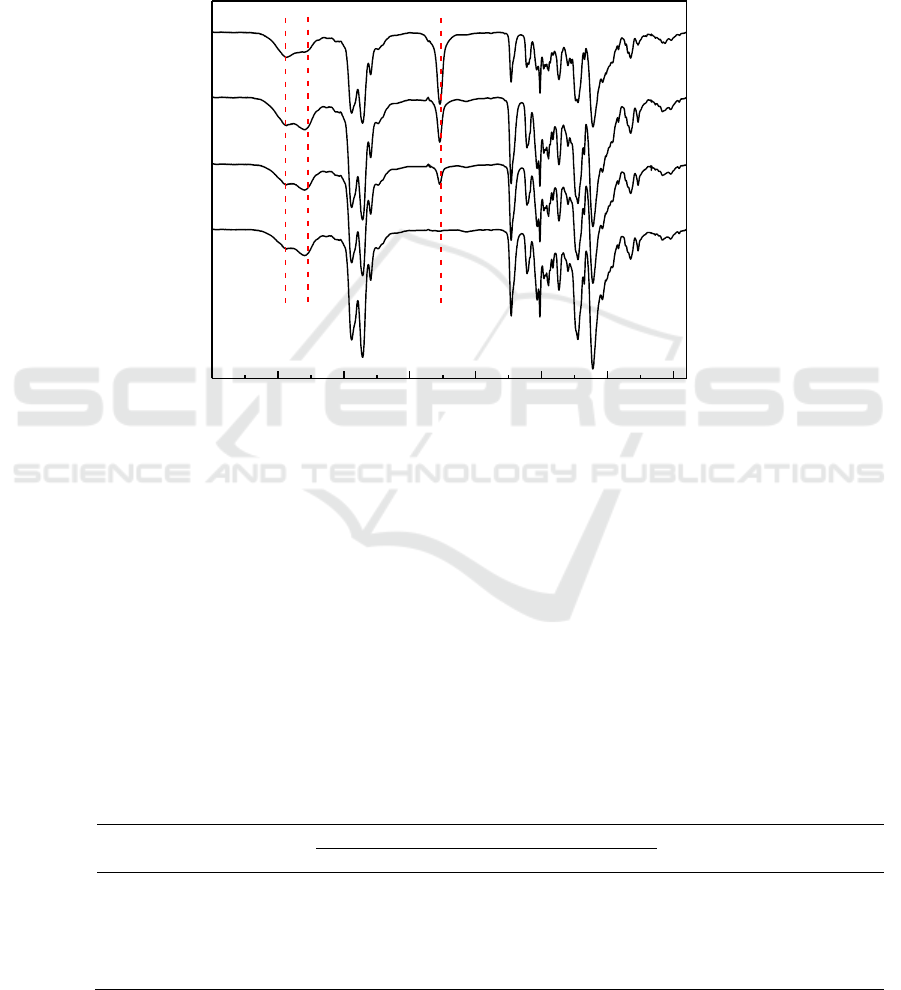
Figure 5 showed the FTIR spectra of the NCO-terminated polyurethane prepolymer reacted with
two equivalents of MPEP for 0, 1, 2 and 3 h at 80°C , respectively. The reaction progress was
indicated by the disappearance of -NCO (2267 cm
-1
) gradually with reaction time, along with the
simultaneous increase of broad N-H peak (3298cm
-1
). The absorption peak of the -NCO group
eventually become zero after 3 h. The results indicated that the alcoholic hydroxyl groups of the
MPEP had completely reacted with the -NCO groups of the polyurethane prepolymers. The targeted
product FEP has been successfully synthesized.
4000 3500 3000 2500 2000 1500 1000 500
3h
2h
1h
Transmittrance(%)
Wave number (cm
-1
)
0h
3464 3298
2267
Figure 5.FTIR spectra of FEP at different time.
The adhesive properties of modified epoxy resin with different FEP contents were studied, and the
results for LSS and T-PS are listed in Table 1. In general, there is a positive correlation between T-PS
and toughness of resin system. For the epoxy systems the LSS at 25°C increased gradually with the
incorporation of FEP but not exceeding 60 percents, the system containing 20 phr FEP showed the
highest LSS value of 31.41 MPa at 70°C . Meanwhile, the T-PS increased abruptly with FEP content
increasing. This phenomenon can be attributed that the molecular interpenetration benefits the
improvement in the compatibility of DGEBA and FEP. As a result, not only does the increased
molecular mass lead to a phase separation, but also that there are lots of strong chemical bonds across
the rubbery phase/resin matrix interface. In other words, an increase of toughness is mainly attributed
to the cavitation or shear-yielding mechanisms [9-11].
Table1.Adhesive properties of FEP/DGEBA/DDM systems.
FEP content, phr
Lap shear strength, MPa
T-Peel strength, kN/m
25°C
70°C
0
31.02
30.43
2.31
20
34.20
31.41
5.03
40
35.21
25.86
6.98
60
37.89
19.74
8.74
80
24.78
6.31
10.37
Preparation of Flexible Epoxy Resin Containing Polyurethane Segments
569

4. Conclusions
In this study, glycidyl-terminated polyurethane prepolymer FEP was prepared and characterized. The
adhesive properties of DGEBA filled with varying wt% of FEP were also investigated. The results
implied that the as-prepared glycidyl-terminated polyurethanes can be utilized for high performance
adhesive and resin fields.
Acknowledgment
This work was supported by the Special Program for Academician Guidance of Heilongjiang
Academy of Sciences, China (NO. 2016-YX-03).
References
[1] Zhang J, Zhang W and Guan D 2016 Polym. Bull. 73 113.
[2] Chaudhary S, Surekha P, Kumar D, Rajagopal C and Roy P K 2015 Polym. Compos. 36 174.
[3] Dittanet P and Pearson R A 2013 Polymer 54 1832.
[4] Qi B, Lu S R, Xiao X E, Pan L L, Tan F Z and Yu J H 2014 Express Polym. Lett. 8 467.
[5] Zaman I, Phan T T, Kuan H C, Meng Q, La L T B and Luong L 2011 Polymer 52 1603.
[6] Dourbash A, Buratti C, Belloni E and Motahari S 2016 J. Appl. Polym. Sci. 134.
[7] Chen D S, Ma C C M, Hsia H C, Wang W N and Lin S R 1994 J. Appl. Polym. Sci. 51 1199.
[8] Liu C, Sun M, Zhang B, Zhang X, Li J and Xue G 2018 J. Appl. Polym. Sci. 135 46458.
[9] Vabrik R, Czajlik I, Túry G, Rusznák I, Ille A and Víg A 2015 J. Appl. Polym. Sci. 68 111.
[10] Li Y and Mao S 1996 J. Appl. Polym. Sci. 61 2059.
[11] Wang H H and Chen J C 1995 Polym. Eng. Sci. 35 1468.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
570
