
The Synthesis of Multi-heteropoly Acid Containing Titanium
and Its Application on Esterification Reaction
J Y Ren, Q Wang
*
, W H Dai, T Liu, J S Yan and Y Feng
Department of biological medicine and chemical Engineering, Liao Ning Institute of
Science and Technology, Benxi, liaoning, 117004, China
Corresponding author and e-mail: Q Wang , 174783285 @qq.com
Abstract. The novel ternary undecatungstotitanotitanic heteropoly acid (H
4
Ti (H
2
O)
TiW
11
O
39
) was synthesized successfully by the method of ion exchanging-concentration. It
was used as the catalyst for the esterification of maleic anhydride with 2-ethylhexyl alcohol in
liquid phase. The effects of reaction time, ratio of anhydride to alcohol and the amount of
water-carrying agent toluene on synthesizing di-2-ethylhexyl maleate were investigated. The
suitable condition for synthesizing the ester was obtained. The catalyst has outstanding
advantages such as high esterification rate, less catalyst consumption.
1. Introduction
Heteropoly acid is a kind of solid super acid, which has been widely used as a new type of catalyst in
organic synthesis reactions. It has many advantages such as high catalytic activity, low equipment
corrosion, and small pollution. It has many reports on its catalytic esterification reaction [1-2].
Maleic acid di-2-ethylhexyl maleate, also known as two ethylhexyl maleate, is abbreviated as
DOM. It is an important internal plasticizer. It is widely used in paint, coatings, adhesives, fiber
treatment agents and so on. The products have the advantages of good gloss, aging resistance, acid
alkali resistance and so on. In addition, it is also widely used in the fields of petroleum and paper
making, is also an important chemical intermediates [3].
O
O
O
+
CH
2
CH(CH
2
)
3
CH
3
HO
C
2
H
5
CH
CH
C
O
O CH
2
CH(CH
2
)
3
CH
3
C
2
H
5
C
O
OH
H
+
C
8
H
17
OH
CH
CH
C
O
O CH
2
CH(CH
2
)
3
CH
3
C
2
H
5
C
O
O CH
2
CH(CH
2
)
3
CH
3
C
2
H
5
At present, the catalyst used in the industrial production of diethylhexyl maleate is still sulfuric
acid. Although sulfuric acid is used as a catalyst, it is inexpensive and easy to obtain, but it requires
high corrosion resistance of equipment, large amount of waste water, and long process flow.
Operation is troublesome, due to long reaction period, high temperature, many side reactions, and
poor product quality. Therefore, the current research on the synthesis of DOM is mainly focused on
the research of catalysts, Wu uses a composite solid superacid SO
4
2-
/ZrO
2
-TiO
2
as a catalyst
[4], Luo
used solid acid catalyst SO
4
2-
/TiO
2
as catalyst [5], Zou Changjun and others used macroporous strong
acidic styrene cation exchange resin catalyst [6]. However, these methods generally have the
Ren, J., Wang, Q., Dai, W., Liu, T., Yan, J. and Feng, Y.
The Synthesis of Multi-heteropoly Acid Containing Titanium and Its Application on Esterification Reaction.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 621-624
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
621

disadvantages of low catalyst activity, large amount, or pressure operation, and high equipment and
operation requirements.
At present, the catalyst used in the industry is still concentrated sulfuric acid, and the development
of a catalyst that can achieve the catalytic effect of concentrated sulfuric acid and overcome its
shortcomings is an urgent problem to be solved. The novel multi-heteropoly acid
undecatungstotitanotitanic acid composite [H
4
Ti (H
2
O) TiW
11
O
39
] was used for the catalytic
synthesis of DOM. The catalytic performance was more excellent. It had the outstanding advantages
of less catalyst and higher esterification rate.
2. Experimental section
2.1. Instruments and Reagents
Experimental instruments include the Beijing Rayleigh WQF-510A FTIR infrared spectrometer and
the Beijing General Analysis TU-1901 double beam UV-Vis spectrophotometer. All reagents used
were of analytical grade.
2.2. Preparation of heteropoly acids
Heteropoly acid catalyst H
4
Ti (H
2
O) TiW
11
O
39
was synthesized according to the literature.
Take 18.15g Na
2
WO
4
2H
2
O and add 100mL deionized water to dissolve it. The pH value of the
ice acetic acid solution is 6.3, heated to the micro tenging, and the side stirring edge slowly drops the
Ti
4+
solution of 0.33 mol/L (TiCl
4
dissolved in the 0.1 mol/L hydrochloric acid) 15mL, and the pH
value is 5.5. After half an hour of reaction, 0.33mol /L Ti
4+
solution was added to 15mL, and the pH
value was adjusted to 5 with glacial acetic acid. After continuing to react to 1.5h, filtering, adding a
proper amount of ethanol into the filtrate and precipitating a colorless oil, after placing the
refrigerator in the refrigerator, separating the oil, adding some deionized water and adding a proper
amount of ethanol to precipitate the oil, after repeated operation and purification for 3 times,
dissolved in 40mL water, and added a proper amount of hydrogen cation exchange resin. The pH
value of the stirring solution is less than 1, and undecatungstotitanotitanic acid can be prepared.
2.3. Esterification
The maleic anhydride 5.0g, undecatungstotitanotitanic acid catalyst 0.1g, a proper amount of 2-
ethylhexyl alcohol, toluene and zeolite are added to the three bottles of 100mL, and the oil water
separator is added to the oil-water separator. The saturated sodium chloride solution is added to the
oil-water separator and heated to the reflux. The reaction has been reacted for a certain time, and the
toluene is steamed out and cooled to room temperature 15 minutes before the end of the reaction.
After that, an appropriate amount of solution was taken and the acid value was determined by
0.05mol/L sodium hydroxide standard solution.
The acid value was determined according to the national standard method, and the esterification
rate was calculated according to the following formula.
esterificationrate =
Acidityvaluebeforereaction − acidvalueafterreaction
Acidityvaluebeforereaction
× 100%
The ester decompressed distillation is refined, and the catalyst is stored in the reactor. The
esterification catalysis of the catalyst under the next same condition is not treated directly. The
catalytic activity is repeated for five times. The catalytic activity has no obvious difference, the
catalyst can be reused many times.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
622
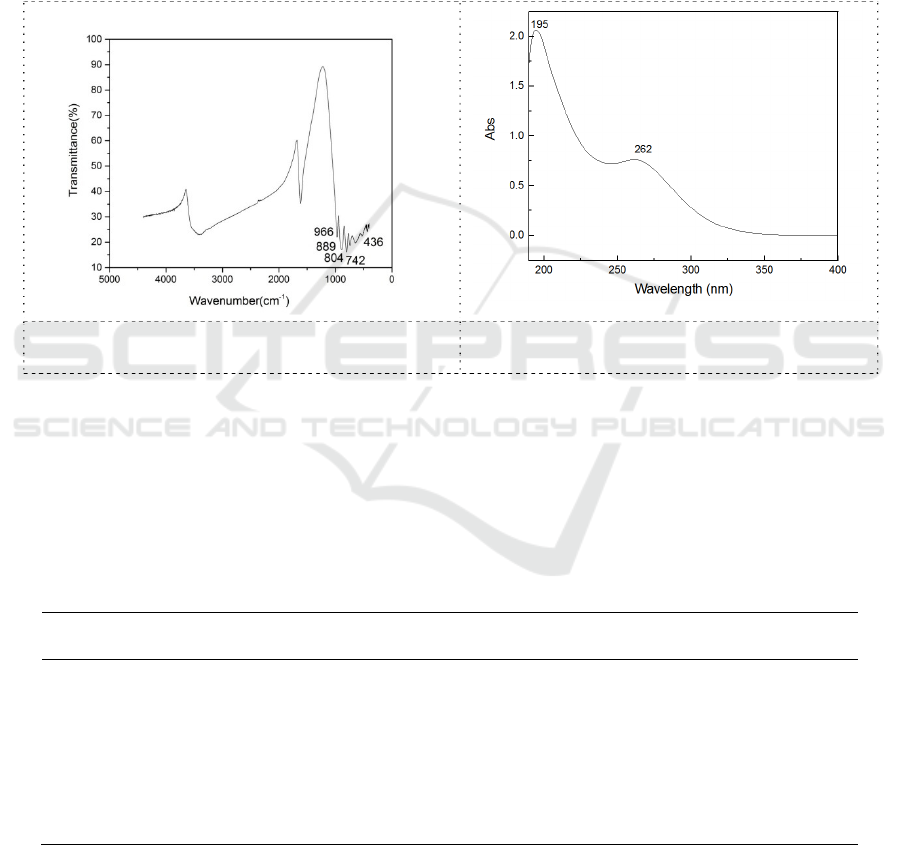
3. Results and discussion
3.1. Infrared and UV spectra of heteropoly acids
Figure 1 and Figure 2 show there are 5 characteristic peaks in the infrared spectrum of the synthetic
products, which conform to the characteristic vibration of Keggin heteropoly acids, respectively: V
Od 966 cm
-1
as terminal oxygen atoms, V Ob 889cm
-1
as co top oxygen atoms, V Oc 804cm
-1
,
742cm
-1
as the oxygen atoms shared by the common side, and V Oa 436 cm
-1
as the oxygen atom
connected with the titanium atom. In the UV spectrum, there are two absorption bands, which are Od
→ W 195 nm and Ob/Oc → W 262 nm, which conform to the characteristic absorption of Keggin
heteropoly acids and be the transition of tungsten oxygen bonds.
Figure 1. infrared spectrum of heteropoly
acid H
4
Ti (H
2
O) TiW
11
O
39
Figure 2. UV spectra of heteropoly acid
H
4
Ti (H
2
O) TiW
11
O
39
3.2. Selection of esterification conditions
For the molar ratio of maleic anhydride to 2- ethylhexanol, the reaction time and the amount of three
conditions for toluene, see Table 1, the optimum reaction conditions are the molar ratio of maleic
anhydride to 2- ethyl hexanol, the reaction time 4 hours, the dosage of toluene with water agent 10ml,
and the esterification rate of two ethyl hexyl maleate to 99.5%.
Table 1.Data of esterification reaction
NO. reaction time (h)
anhydride alcohol
ratio (mol)
Toluene (mL)
esterification rate
(%)
1 3 3 15 84.3
2 4 3 15 93.8
3 5 3 15 95.7
4 4 2.5 15 86.8
5 4 3.5 15 96.3
6 43594.7
7 4 3 10 99.5
8 4 3 20 88.6
Maleic anhydride (maleic anhydride) is a highly active acylate, which reacts with 2- ethyl hexanol
to produce monoester quickly. One carboxyl group is retained in the mono ester molecule, which can
continue to react with 2- ethylhexyl alcohol to produce diester, but the acylation activity of
carboxylic acid is weak and needs to be carried out in the presence of acid catalyst. Heteropoly acid
The Synthesis of Multi-heteropoly Acid Containing Titanium and Its Application on Esterification Reaction
623

as catalyst has mild reaction conditions, high esterification rate, and can be reused. [10] This is
determined by the characteristics of heteropoly acid catalyst. The esterification reaction of carboxylic
acid is reversible. In order to improve the conversion of reversible reaction, the amount of 2- ethyl
hexanol in reactant can be increased, but excessive excess makes the cost increase. The better method
is to reduce the concentration of the product and add the toluene with water agent, which can make
the reaction water leave the reaction system and get a higher conversion rate.
4. Conclusions
The synthesis of two ethyl hexyl maleate with undecatungstotitanotitanic acid as catalyst was used as
a catalyst for the synthesis of two ethyl hexyl maleate. It was easy to operate, and a good
esterification rate was obtained. The catalytic activity was high and the amount of catalyst was less. It
could be reused continuously. No waste acid was discharged and there was no environmental
pollution. It was of positive significance to the solution of the existing sulfuric acid catalytic process.
Acknowledgments
This work was financially supported by Liaoning Natural Science Foundation Project (20170540475)
and Science and technology research project of Liaoning Provincial Education Department
(201710311) and entrepreneurial training program for college students of Liaoning Institute of
Science and Technology in 2018 fund (201811430071) (201811430073).
References
[1] Erythropel H C, Brown T, Maric M, Nicell J A, Cooper D G and Leask R L. 2015 Designing
greener plasticizers: Effects of alkyl chain length and branching on the biodegradation of
maleate based plasticizers [J]. Chemosphere, 134-139.
[2] Yamak H B, Tamer Y and Yıldırım H. 2016 The effect of maleic acid diesters type on the
stability of vinyl acetate emulsion polymers [J]. Colloids and Surfaces A: Physicochemical
and Engineering Aspects, 497-502.
[3] Butini S, Nikolic K, Kassel S, Brückmann H, Filipic S, Agbaba D, Gemma S, Brogi S,
Brindisi M, Campiani G and Stark H. 2016 Polypharmacology of dopamine receptor
ligands [J]. Progress in Neurobiology, 142-149.
[4] Wu H T, Yu C and Ge S X. 2007 Solid superacid SO
4
2-
/ZrO
2
-TiO
2
catalyzed synthesis of [J].
chemical and bioengineering of maleic acid two (2- ethyl) hexyl ester, 24 (5): 21 ~ 23.
[5] Luo Z C, Wang W T and Ding S G. 2004 Solid acid catalyzed synthesis of maleic acid (2-
ethyl hexyl ester) [J]. Tianjin chemical industry, 18 (5): 11 ~ 12.
[6] Zou C J, Kong Z H and Yang H R. 1995 Catalytic synthesis of maleic acid two di-2-ethylhexyl
maleate [J]. Jilin technology, 3: 26-29.
[7] Cheryl-Low Y L, Lee H V and Abd Hamid S B. 2017 Catalytic upgrading of bio oil model
compound into polyol ester via green alginate catalyzed esterification reaction [J]. Process
Safety and Environmental Protection, 111-120.
[8] Chen L F, Zhang Z C, Lu Y J, Zhang C, Zhang X, Zhang C Q and Roskilly A P. 2017
Experimental study of the gaseous and particulate matter emissions from a gas turbine
combustor burning butyl butyrate and ethanol blends [J]. Applied Energy, 195-206.
[9] Caplar R and Kulisic P. 1973 Proc. Int. Conf. on Nuclear Physics (Munich) vol 1 Amsterdam:
North-Holland/American Elsevier, p 517
[10] Hu G D and Wu Q Y. 1991 Catalytic characteristics of heteropoly acid - pseudo liquid phase
catalysis [J]. Liaoning chemical industry, 4, 14 ~ 19.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
624
