
Fabrication of Core-shell Structure Nanocomposites of Gold
Nanoparticles@METAC
Sujuan Liu
1*
,Zhaoyu Wu
1
1
Department of Chemistry and Industry,Mudanjiang Normal University, Aiminjie,Mudanjiang , China
Keywords: Core-shell structure, nanocomposites, gold nanoparticles.
Abstract: Novel core-shell structure nanocomposites are fabricated by grafting polymers of poly([2-
(Methacryloyloxy)ethyl]trimethylammonium chloride) onto gold nanoparticles through surface-initiated,
atom-transfer radical polymerization (ATRP) in 2-propanol/water mixed solvents. The surface of citrate-
stabilized gold nanoparticles was first modified by a disulfide initiator for ATRP. The Au@polymer
nanocomposites display a well-defined core/shell nanostructure that were characterized by TEM, FTIR,XPS
and UV-visible spectroscopy. Such core-shell nanocomposites can be considered as water-dispersible
nanotanks for hydrophobic drugs in the development of multifunctional biodelivery systems
1 INTRODUCTION
Metal nanoparticles that are imbedded in polymer
composites have been of intense recent interest [1]
with regard to their fabrication and potential
application in areas such as electronics, optics,
magnetics, catalysts, and sensors [2-7]. As a well-
known noble metal, gold is widely investigated due
to its specific impact in the fields of biotechnology
and bioscience [8-14]. A large number of polymer
molecules have been selected to decorate the surface
of gold nanoparticles in physical or chemical
manners for different purposes [15-20].
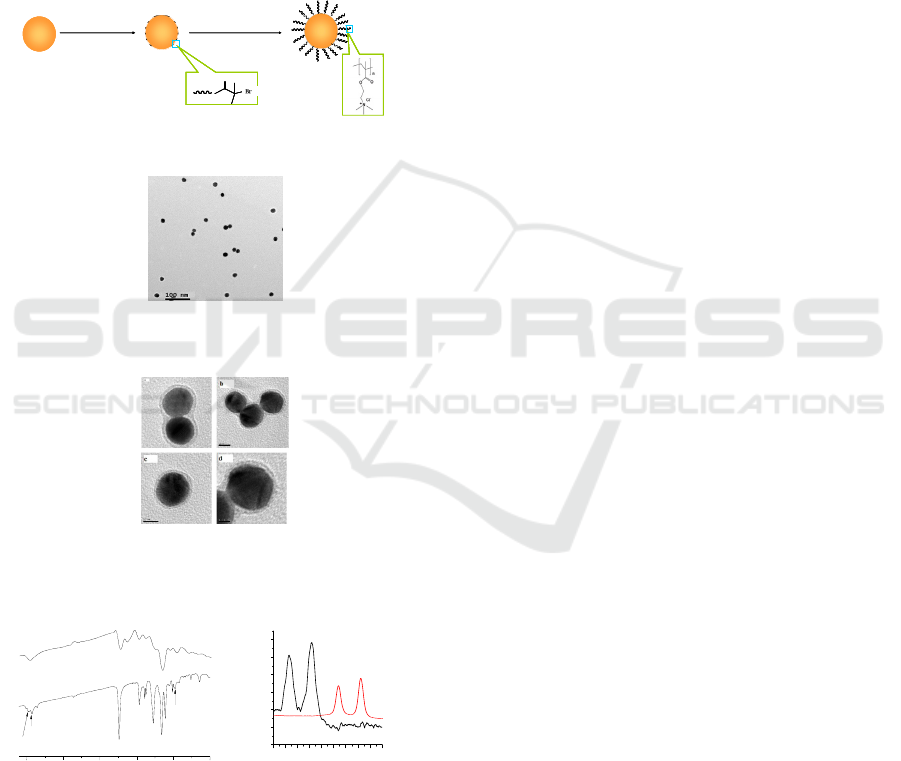
In this paper, we report the fabrication of
METAC modified gold nanoparticles as
nanocomposite materials by SI-ATRP on a gold
surface. The typical experimental procedure is
illustrated in Scheme 1. First, the disulfide initiator
was immobilized on the surface of gold
nanoparticles (Au@initiator). Subsequently, ATRP o
[ 2-(Methacryloyloxy)ethyl ] trimethylammonium
chloride (METAC) occurred on gold nanoparticles
catalyzed by N,N,N′,N′,N′′-
pentamethyldiethylenetriamine (PMDETA) and
CuBr. Such environmentally responsive
nanocomposites provide a smart supporter or carrier
to transition metal ions and nanoparticles to
construct novel bimetallic nanocomposites,
especially in catalyst applications.
2 MATERIALS AND METHODS
2.1 Materials
The disulfide initiator [ S-(CH
2
)
2
-OCOC(CH
3
)
2
Br
]
2
, [ 2-(Methacryloyloxy)ethyl ]
trimethylammoniumchloride(METAC),N,N,N’,N’,N
’’-pentamethyl-diethylenetriamine (PMDETA) were
purchased from Sigma Aldrich. CuBr, HAuCl
4
,
sodium citrate, and other chemicals were obtained
from. METAC was purified to remove the inhibitor.
The gold nanoparticles with an average diameter of
20 nm were prepared from HAuCl
4
and sodium
citrate by conventional citrate-reduction methods
[21].
2.2 Instrumentation
FTIR and UV-vis spectra, respectively, were
recorded on a Excalibur HE 3100 instrument
(Varian) and a U-4100 UV-vis spectrometer
(HITACHI). TEM images were obtained by a
Tecnai G
2
F30 transition electron microscope (FEI).
X-ray photoelectron spectroscopy (XPS) was
performed on an K-Alpha electron spectrometer
(Thermofisher Scienticfic Company) using 300 W
Al Kα radiation at about 1×10
-8
mbar,and the

binding energies were referenced to the C1s line at
284.8eV from adventitious carbon.
2.3 Preparation of SI-ATRP Initiator
The initiator immobilized on gold nanoparticles
(Au@initiator) was prepared from fresh gold
nanoparticles through ligand exchange between
disulfide initiator and citrates. Generally, a certain
volume of fresh gold nanoparticles in water was
slowly added to same volume of a N,N-
dimethylformamide (DMF) solution that dissolved
3.0 mM initiator with stirring for 24 h. The
Au@initiator was collected and washed with DMF
and Millipore purified deionized water by
centrifugation. Finally, Au@initiator was dispersed
in DMF and stored at 20 °C under an argon
atmosphere.
2.4 SI-ATRP Process of METAC
Polymerization of METAC on a gold surface was
performed in a mixed solvent at ambient conditions
according to the literature [22-25]. In detail, a round-
bottom flask was added with CuBr (28.6 mg) and
degassed by three freeze pump thaw cycles under N
2
atmosphere. A degassed mixture of Au@initiator (2
mg) dissolved in DMF (2 mL), PMDETA
(0.208mL), 2-propanol (0.5 mL), and a certain
amount of free initiator (0.5-20 mg) was injected
into the flask through a syringe, followed by
degassed METAC (1.05-2.10 g) with drastic stirring.
The reaction was performed for 24 h and terminated
by opening the system to air. The Au@METAC
nanocomposites were purified by more than three
cycles of centrifugation, 2-propanol/DMF washing.
3 RESULTS AND DISCUSSION
3.1 Preparation and Component Analysis
Gold nanoparticles with diameters of 20 nm were
synthesized by using the conventional citrate-
reduction method and can be easily dispersed in
warter without aggregation, as shown in Figure 1.
The immobilization of disulfide initiator on the gold
surface was achieved through ligand exchange
between disulfide and citrate. The ATRP reaction of
METAC from a free initiator or a macromolecular
initiator was suggested to be carried out in a protic
alcohol solvent [26-27].
Herein, the solvent is mixed
protic 2-propanol with DMF according to the
solubility of the Au@initiator and METAC chains.
A large amount of PMDETA was used to maintain
the activity of the catalytic system, and an appreciate
amount of free initiator was added into the reaction
mixture to control the polymerization [28]. Figure
3A shows the FT-IR spectra of Au@initiator and as-
prepared Au@METAC nanocomposites. The profile
of the Au@initiator (curve b) is similar to that of the
pure disulfide initiator, whose characteristic
absorbance at 2922, 2850 (CH
2
stretching), and 1729
cm
-1
(ester carbonyl stretching) of Au@initiator
(curve b) denotes the presence of the ATRP initiator
on gold nanoparticles. For obtained Au@METAC
(curve a), the characteristic peaks at 2968, 1730, and
1457 cm
-1
represent the CH
3
and CH
2
stretching
vibrations, ester carbonyl stretching, -CH
2
- N
+
(CH
3
)
3
bending vibrations according to the references
[22,23]. 960cm
-1
(N
+
(CH
3
)
3
),1166,1278cm
-1
(C-O). It
shows that the polymerization of METAC is
performed successfully. So, the FT-IR results reveal
that the Au@METAC nanocomposites are easily
protonated by hydrochloride.
Moreover, the Au@METAC nanocomposite
samples and pure METAC (sigma, as a comparison)
were analyzed by X-ray
photoelectronspectroscopy(XPS).The element
components of Au@METAC nanocomposites are
approximately contributed to C (41.2%), N (2.5%),
O (11.6%), Au (43.4%), and Cl (1.3%). Figure 3B
shows the binding-energy decrease of Au 4f within a
range of 8.1 eV and 8 eV (95.4 to 87.3 eV for Au
4f5/2 and 91.6 to 83.6 eV for Au 4f7/2) after (a) and
before (b) gold nanoparticle disulfide initiator
modification by METAC. This proves the formation
of Au-C bonds on the gold surface.
All of these
component analyses demonstrate that the designed
Au@METAC nanocomposites were successfully
obtained from a consecutive ATRP reaction.
3.2 Morphology and Structural
Characterizations
Figure 2 show the transmission electron microscopy
(TEM)images of Au@METAC nanocomposites
under different magnifications. The well-dispersed
nanostructures consist of a gold “core” with an
average diameter of 20 nm and a polymer “shell”
about 10 nm thick, which could be clearly observed
after staining by phosphotungstic acid.
4 CONCLUSION
In summery, we have demonstrated that SI-ATRP of
METAC can be performed on the surface of initiator
modified gold nanoparticles to fabricate
Au@polymer nanocomposites. The as-prepared
Au@METAC nanocomposites have a distinct core
shell nanostructure with gold cores and polymer
shells. The polymer “shell” has a network scaffold.
The biocompatible and amphiphilic core-shell
nanostructures can be considered as water-
dispersible nanotanks for hydrophobic drugs, which
may have great potential in the multifunctional
biodelivery of hydrophobic drugs. The fabrication
strategy for core-shell nanostructures can be
generalized to prepare novel structured materials in
nanotechnology and biotechnology.
METAC
CuBr/PMDETA
2-propanol/H
2
O
Au@initiator Au@METAC
Citrate-stabilized
gold nanopartiacles
Au
Au
s
o
O
Au
DMF/H
2
O
Disulfide initiator
METAC
CuBr/PMDETA
2-propanol/H
2
O
METAC
CuBr/PMDETA
2-propanol/H
2
O
Au@initiator Au@METAC
Citrate-stabilized
gold nanopartiacles
Au
Citrate-stabilized
gold nanopartiacles
AuAu
Au
s
o
O
AuAu
s
o
O
s
o
O
AuAuAu
DMF/H
2
O
Disulfide initiator
DMF/H
2
O
Disulfide initiator
Scheme1. Au@METAC fabrication
Figure 1.TEM image of GNP dispersed in THF. Scale
bar:100 nm.
Figure 2. TEM images of Au@METAC nanocomposites
dispersed in water.
(A) (B)
Figure 3. (A) FT-IR spectra of Au@initiator (a) and
Au@METAC nanocomposites washed by 2-
propanol,(B)Au 4f spectra from XPS analysis of
Au@initiator (a) and Au@METAC nanocomposites (b).
ACKNOWLEDGEMENTS
This research is supported by the research projects
(No. 201710233007,G2017e2447andQN201604).
REFERENCES
1. Mayer, A. B.; Mark, J. E.; Morris, R. E. Palladium and
platinum nanocatalysts protected by amphiphilic block
copolymers. Polym. J. 1998, 3, 197-205.
2. Hostetler, M. J.; Zhong, C. J.; Yen, B. K. H.;
Anderegg, J.; Gross, S.M.; Evans, N. D.; Porter, M.;
Murray, R. W. Stable, Monolayer-Protected Metal
Alloy Clusters. J. Am. Chem. Soc. 1998,120, 9396-
9397.
3. Liu, S.; Zhang, Z.; Han, M. Nanometer-Sized Gold-
Loaded Gelatin/Silica Nanocapsules. Ad . Mater.
2005, 17, 1862-1866.
4. Zhu, H. F.; Tao, C.; Zheng, S. P.; Li, J. B. One step
synthesis and phase transition of phospholipid-
modified Au particles into toluene. Colloids Surf., A
2005,257, 411.
5. Bastys, V.; Pastoriza-Santos, I.; Rodrı ´guez-Gonza
´lez, B.; Vaisnoras,R.; Liz-Marza ´n,L.M. Formation
of Silver Nanoprisms with Surface Plasmons at
Communication Wavelengths. Ad . Funct. Mater.
2006, 16, 766-773.
6. Zhu, H. F.; Tao, C.; Zheng, S. P.; Wu, S. K.; Li, J. B.
Effect of alkyl chain length on phase transfer of
surfactant capped Au nanoparticles across the
water/toluene interface. Colloids Surf.,A 2005, 256,
17-20.
7. Dong, H.; Fey, E.; Gandelman, A.; Jones, W. E.
Synthesis and assembly of metal nanoparticles on
electrospun poly(4-vinylpyridine) fibers and poly(4-
vinylpyridine) composite fibers. Chem. Mater.
2006,18, 2008-2011.
8. Vossmeyer, T.; Guse, B.; Besnard, I.; Bauer, R. E.;
Mullen,K.;Yasuda,Gold Nanoparticle/Polyphenylene
Dendrimer Composite Films: Preparation and Vapor-
Sensing Properties. A. Adv. Mater. 2002, 14, 238-242.
9. Storhoff,J.J.;Elghanian,R.;Mucic,R.C.;Mirkin,C.A.;Le
tsinger,R. L. One-Pot Colorimetric Differentiation of
Polynucleotides with Single Base Imperfections Using
Gold Nanoparticle Probes. J. Am. Chem. Soc. 1998,
120, 1959-1964.
10. Wohltjen, H.; Snow, A. W. Colloidal metal- insulator-
metal ensemble chemiresistor sensor. Anal. Chem.
1998, 70, 2856-2859.
11. Demaille, C.; Brust, M.; Tsionsky, M.; Bard, A.
Fabrication and characterization of self-assembled
spherical gold ultramicroelectrodes, J. Anal.
Chem.1997, 69, 2323-2328.
12. Al-Rawashdeh, N. A. F.; Sandrock, M. L.; Seugling,
C. J.; Foss,C. A. J. Visible Region Polarization
Spectroscopic Studies of Template-Synthesized Gold
Waven
98 96 94 92 90 88 86 84 82 80
600
800
1000
1200
1400
1600
1800
Au4f
7/2
83.6
87.3
b
In te n s ity
Binding Energy/ev
a
95.4
91.6
Au4f
5/2

Nanoparticles Oriented in Polyethylene. J. Phys.
Chem. B 1998, 102, 361-371.
13. Evans, S. D.; Johnson, S. R.; Cheng, Y. L.; Shen, T.
Vapour sensing using hybrid organic-inorganic
nanostructured materialsJ. Mater.Chem. 2000, 10,
183-188.
14. Maier, S.A.;Brongersma,M.L.;Kik,P.G.;Meltzer,
S.;Requicha,A. A. G.; Atwater, H. A. Plasmonics a
route to nanoscale optical devices. Adv. Mater. 2001,
13, 1501-1505.
15. Wuelfing, W. P.; Gross, S. M.; Miles, D. T.; Murray,
R. W. Nanometer Gold Clusters Protected by Surface-
Bound Monolayers of Thiolated Poly(ethylene glycol)
Polymer Electrolyte J. Am.Chem. Soc. 1998, 120,
12696-12697.
16. Teranishi, T.; Kiyokawa, I.; Miyake, M. Synthesis of
Monodisperse Gold Nanoparticles Using Linear
Polymers as Protective Agents. Ad . Mater. 1998, 10,
596.
17. Lowe, A. B.; Sumerlin, B. S.; Donovan, M. S.;
McCormick, C. L. Facile Preparation of Transition
Metal Nanoparticles Stabilized by Well-Defined
(Co)polymers Synthesized via Aqueous Reversible
Addition-Fragmentation Chain Transfer
Polymerization. J.Am. Chem. Soc. 2002, 124, 11562-
11563.
18. Jordan, R.; West, N.; Ulman, A.; Chou, Y.; Nuyken,
O. Nanocomposites by surface-initiated living cationic
polymerization of 2-oxazolines on functionalized gold
nanoparticles. Macromolecules 2001, 34, 1606-1611.
19. Watson, K. J.; Zhu, J.; Nguyen, S. T.; Mirkin, C. A.
Hybrid Nanoparticles with Block Copolymer Shell
Structures. J. Am. Chem.Soc. 1999, 121, 462-463.
20. Raula, J.; Shan, J.; Nuopponen, M.; Niskanen, A.;
Jiang, H.;Kauppinen, E. I.; Tenhu, H. Synthesis of
Gold Nanoparticles Grafted with a Thermoresponsive
Polymer by Surface-Induced Reversible-Addition-
Fragmentation Chain-Transfer Polymerization.
Langmuir 2003, 19, 3499-3504.
21. Link, S.; El-Sayed, M. A. Spectral properties and
relaxation dynamics of surface plasmon electronic
oscillations in gold and silver nanodots and nanorods.
J. Phys. Chem. B 1999, 103, 8410-8426.
22. Hao, E.; Lian, T. Layer-by-Layer Assembly of CdSe
Nanoparticles Based on Hydrogen Bonding. Langmuir
2000, 16, 7879-7881.
23. Ruokolainen, J.; Brinke, G.; Ikkala, O.; Torkkeli, M.;
Serimaa, R. Mesomorphic Structures in Flexible
Polymer-Surfactant Systems due to Hydrogen
Bonding. Macromolecules 1996, 29, 3409-3415.
24. Cui, Y.; Tao, C.; Zheng, S. P.; He, Q.; Ai, S. F.; Li, J.
B. Synthesis of Thermosensitive PNIPAM-co-MBAA
Nanotubes by Atom Transfer Radical Polymerization
within a Porous Membrane. Macromol.Rapid
Commun. 2005, 26, 1552-1556.
25. Cui, Y.; Tao, C.; Tian, Y.; He, Q.; Li, J. B. Synthesis
of PNIPAM-co-MBAA Copolymer Nanotubes with
Composite Control. Langmuir 2006, 22, 8205-8208.
26. Xia, J.; Zhang, X.; Matyjaszawski, K. Atom Transfer
Radical Polymerization of 4-Vinylpyridine.
Macromolecules 1999, 32, 3531-3533.
27. Vidts, K. R. M.; Prez, F. E. D. Design of water-
soluble block copolymers containing poly(4-
vinylpyridine) by atom transfer radical
polymerization. Eur. Polym. J. 2006, 42, 43.
28. Li, D. J.; Sheng, X.; Zhao, B. Environmentally
responsive "hairy" nanoparticles: Mixed homopolymer
brushes on silica nanoparticles synthesized by living
radical polymerization techniques. J. Am. Chem. Soc.
2005, 127, 6248-6256.
