
Green Microwave Synthesis of Cuprous Oxide
Microparticles And the Photocatalytic Degradation Properties
Weichao Li
1
, Pen Deng
1
, Liexing Zhou
2
, Linkun Xie
1
and Xijuan Chai
1
1
Yunnan Provincial Key Laboratory of Wood Adhesives and Glued Products, Southwest Forestry University, Kunming,
China;
2
Research Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming, China;
Keywords: Cuprous oxide; Microwave synthesis; Microparticles; Photocatalytic degradation properties.
Abstract: Cu
2
O microparticles with three different morphology were prepared by a green, facile and additive free
microwave synthesis method with household common microwave oven. The morphology of cuprous oxide
can be easily controlled by adjusting the amount of glucose added, via which the sphere, cube and
octahedron cuprous oxide were synthesized. The phase, morphologies and optical properties of cuprous
oxide were characterized by XRD, SEM and UV-vis. The photocatalytic efficiency are evaluated by
monitoring the photocatalytic degradation of Methylene blue (MB) solution under visible light irradiation.
The results show that the spherical, cubic and octahedral cuprous oxide exhibited the high degradation
efficiency.
1 INTRODUCTION
Cuprous oxide (Cu
2
O) is a typical P-type
semiconductor material with a band gap of
approximately 2.17 eV, with unique optical and
electrical properties and photoelectrochemically
properties[S. Kakuta et al.,2009;J.Y. Kim et al.,
2009 and D.J. Norris et al. 2001]. With the
development of nanotechnology, Cu
2
O nanoparticles
have widespread applications in solar energy
conversion, photo catalytic degradation of organic
pollutants and catalysis in organic synthesis[D.J.
Norris et al. 2001].
Cu
2
O nanoparticles can be prepared by the solid-
phase synthesis, liquid-phase method and gas-phase
method[H. Pang et al,2010; P. Liu et al.,2011 and
K.D. Bhatte et al.,2010]. Comparatively speaking in
the liquid-phase method, the chemical deposition
method require relatively simple experimental
conditions and are easy to operate, which is often
used. However, in order to obtain nanoparticles with
uniform morphology and particle size, most of the
chemical deposition methods need to use drastic
synthesis conditions like high temperature and
pressure, use of toxic reagents, long reaction time,
and requirement of external additives during the
reaction[F.K. Liu et al.,2004; K.D. Bhatte et al.,2012;
F.K. Liu et al.,2008; G.C. Xi et al.,2008].
Currently microwave assisted synthesis attracts
more attention owing to its advantages like
volumetric heating, fast kinetics, selectivity, less
energy requirements and time economy[QingweiZhu
et al.,2011; Manohar A. et al.,2013]. There are also
some studies about preparation of cuprous oxide
powders by microwave methods. However,
microwave method prepared Cu
2
O nanocomposites
reported in the literature need either the addition of
surfactants[QingweiZhu et al.,2011] or use of toxic
reducer [Manohar A. et al.,2013], or microwave
irradiation time is as long as 0.5-1 hours, or require
special microwave reaction equipment[E. Lu
Hipolito et al.,2017; Jun Liu et al.,2009]. Therefore,
it is still a challenge to develop simple and
controllable routes for the visible-light responsive
Cu
2
O with excellent photocatalytic performance via
microwave assisted synthesis.
Based on the above considerations, this work
described a facile and additive free microwave
method for creating high photocatalytic activity
Cu2O, which synthesize three types of cuprous
oxide microcrystals with household common
microwave oven. The phase, morphologies and
optical properties of three types of cuprous oxide
were characterized by XRD, SEM, and UV-vis.

Their photocatalytic activities are determined by
monitoring the photocatalytic degradation of MB.
2 EXPERIMENT
2.1 Synthesis of Cu
2
O Nanoparticles
Nanocrystalline formation was carried out in
domestic Galanz microwave oven operating at 180
W and frequency of 2.45 GHz. In a typical Cu
2
O
synthesis process, 15ml aqueous solution of sodium
hydroxide (4M) was added slowly into 200ml
aqueous solution of CuSO
4
•5H
2
O (0.1 M) in a
beaker at room temperature (the whole process lasts
for 2min) while stirring for 30min. The calculated
volume of glucose solution (1.0 M) was slowly
added into the above solution and then placed in a
microwave oven. Reaction was performed at 180 W
for 10 minutes. The products were collected by
centrifugation, washed with deionized water and
absolute ethanol, and dried in a vacuum at 50 °C for
24 h.
2.2 Method of Characterization
The morphologies were examined using SEM on a
Hitachi S-4800. The formed Cu2O powder was then
characterised by X-ray diffraction (XRD) (Brukar
D8 advance X-ray diffractometer using
CuKα=1.54060 Å) with scanning 2 theta (θ) angle
ranging from 15° to 100°. UV–vis diffuse
reflectance spectra were obtained by UV–vis
spectrophotometer equipped with an integrated
sphere (TU-1901, China). BaSO4 was used as a
reference for the measurements.
2.2 Photocatalytic Reaction
0.2 g sample of Cu
2
O was dispersed in 10 mg/L MB
solution at 25 ◦C in a 250ml quartz reactor and was
illuminated with a 250 W tungsten lamp. Before
irradiation, the suspensions were sonicated in the
dark for 30 min to make the powder disperse well in
the solution. After that, 1ml of H
2
O
2
was added into
above suspension and then the lamp was turned on
to conduct the photocatalytic reaction at room
temperature while magnetic stirring was kept all
along with the reaction. At regular intervals, 10 ml
of the suspension was sampled and separated by
centrifugation at 8000 rpm for 10 min. The
concentration of remaining pollutant was measured
by its absorbency (A) at 484 nm with a Hitachi UV-
3010 spectrophotometer[P. Chen et al.,2004].
3 EXPERIMENT
3.1 Characterization of Prepared
Cu2O
Figure 1: SEM images of the Cu2O samples with different
morphologies.
(a)octahedron, (b) cube, (c) sphere and cube,(d) sphere
Fig. 1 shows the morphology of Cu
2
O sample
prepared at the molar ratio of glucose solution to
copper sulfate of 0.6, 1.0, 1.25 and 2.0 respectively.
Fig.1(a) shows most of the crystals are octahedral
shape with the size about 200-400 nm. Fig.1(b)
discloses cubic shape crystal with the size about 0.8-
1um. The spherical crystals are shown in Fig.1(d)
with the particle size about 200-350nm. The crystals
shown in Fig.(c) have a spherical and square mixed
crystals. As can be seen from Fig.1, the three types
of cuprous oxide particles are well dispersed without
any particles aggregate. The amount of reducing
agent glucose determines the saturation of the Cu
+
in
the system, which determines the mode growth and
the final morphology of the cuprous oxide
nuclei[Xiaoyan Zhou et al.,2014]. The SEM results
the morphology of cuprous oxide should can be
controlled by adjusting the amount of glucose added.
The XRD patterns of four kinds Cu
2
O in Fig. 2
shows that all diffraction peaks, they are (110),
(111), (200), (220), (311) and (222) planes, which
can match well with the standard Joint Committee
on Powder Diffraction Standards (JCPDS) card No.
78-2076. No impurity peaks were observed in this
pattern, which fully demonstrates that the
1um
(c)
500nm
(d)
1um
(b)
500nm
(a)
crystallinity of Cu
2
O is very good and the purity is
high.
The ultraviolet absorption spectra of Cu
2
O
powders was shown in Fig.3, which indicates that
three prepared samples exhibit broad and strong
absorption peaks in the visible region. The sphere,
cube and octahedron Cu
2
O has the maximum
absorption peak at 511nm, 512 nm, and 495 nm
respectively. The forbidden band width of cuprous
oxide is calculated according to a classical
semiconductor formula αEP=K(EP-Eg)
1/2
. The band
gaps calculated for spheres, cubes, and octahedron
Cu
2
O were 2.42 eV, 2.42 eV, and 2.50 eV,
respectively, which corresponds to a typical
nanosize Cu
2
O[W. Wang et al.,2011].
Figure 2: XRD pattern of different morphology of Cu
2
O.
(a) sphere (b)octahedron;(c) cube;(d) mixed shape
Figure3:UV–vis absorption spectra of Cu2O .
(1) sphere (2)octahedron;(3) cube;
3.2 Photocatalytic degradation of MB
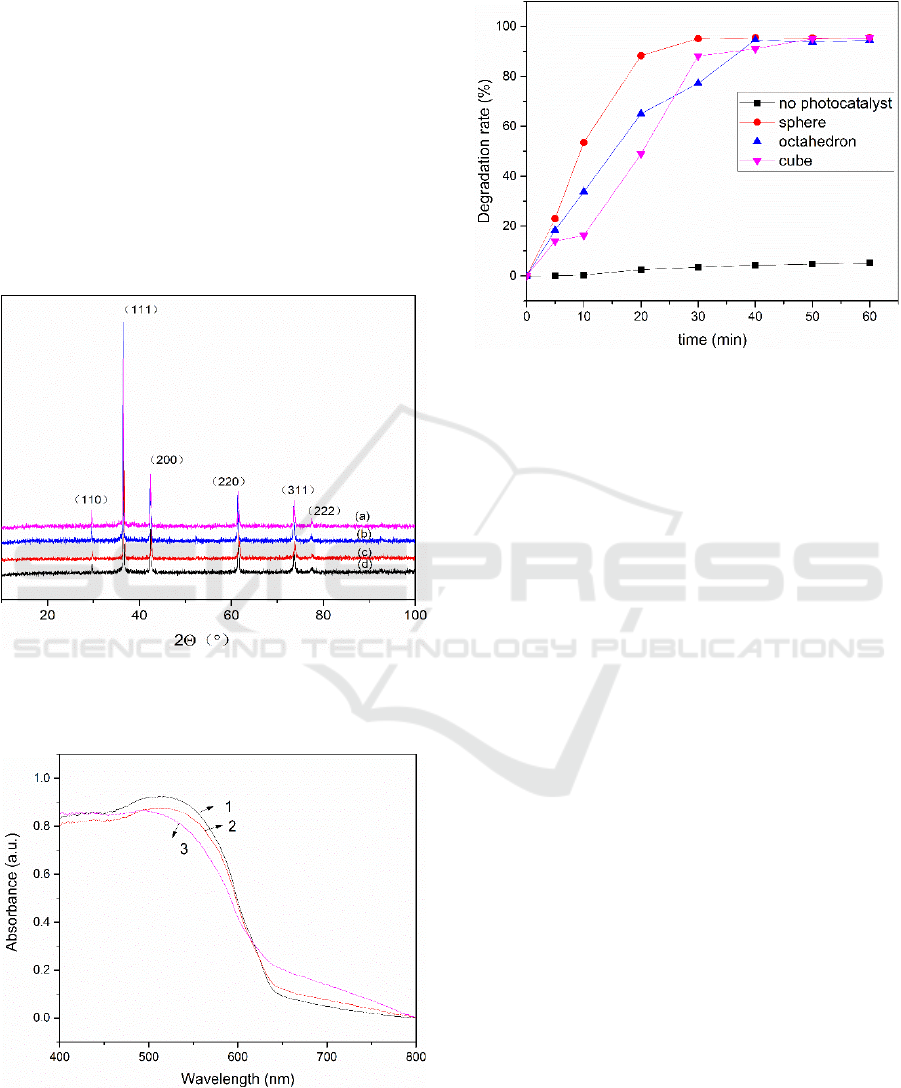
Figure 4:Catalytic degradation of MB curves byCu2O.
The photocatalytic abilities of cuprous oxide are
evaluated by the photocatalytic discoloration of
10mgL
-1
MB as shown in Fig.4. It can be seen that
direct decomposition of MB without cuprous oxide
is pretty low. As for the individual cuprous oxide, it
can be found that sphere Cu
2
O shows the highest
photocatalytic activity with the almost complete
discoloration at the beginning 30min. This is due to
the fact that spherical Cu
2
O has a relatively large
specific surface area and can adsorb more substances
at the beginning. However, when the degradation
time exceeded 30 minutes, the cubic and octahedral
cuprous oxide exhibited the same high efficiency of
degradation as the spherical cuprous oxide.
4 CONCLUSIONS
Sphere, cube and octahedron cuprous oxide were
synthesized by a facile and additive free microwave
synthesis with household common microwave oven
via adjusting the amount of glucose. The phase,
morphologies and optical properties of three types of
cuprous oxide were characterized by XRD, SEM,
and UV-vis. SEM images indicate the three types of
cuprous oxide particles are well dispersed without
any particles aggregate. XRD and UV-vis spectrum
show s all diffraction peaks of the three different
morphology Cu
2
O can match well with the
standardCu
2
O peak and all of the Cu
2
O exhibit broad
and strong absorption peaks in the visible region.
The results of photocatalytic efficiency show that the
spherical, cubic and octahedral cuprous oxide
exhibited the high degradation efficiency.

ACKNOWLEDGEMENTS
This work was supported by the National Natural
Science Foundation of China (No. 31660179), Key
Laboratory of Wood Adhesives and Adhesive
Products in Yunnan Province Open Fund(201502)
REFERENCES
1. S. Kakuta, T. Abe, Photocatalytic activity of Cu
2
O
nanoparticles prepared through novel synthesis
method of precursor reduction in the presence of
thiosulfate, Solid State Sciences 11 (2009) 1465–1469.
2.
J.Y. Kim, J.C. Park, A. Kim, A.Y. Kim, H.J. Lee, H.
Song, K.H. Park, Cu
2
O nanocube-catalyzed cross-
coupling of aryl halides with phenols via Ullmann
coupling,European Journal of Inorganic Chemistry
(2009) 4219–4223.
3.
D.J. Norris, Y.A. Vlasov, Chemical approaches to
three-dimensional semiconductor photonic
crystals,Adv. Mater. 13 (2001) 371–376.
4.
H. Pang, F. Gao, Q. Lu, Glycine-assisted double-
solvothermal approach for various cuprous oxide
structures with good catalytic activities,
CrystEngComm12 (2010) 406–412.
5.
P. Liu, Z. Li, W. Cai, M. Fang, X. Luo, Fabrication of
cuprous oxide nanoparticles by laser ablation in PVP
aqueous solution, RSC Advances 1 (2011) 847–851.
6.
K.D. Bhatte, P. Tambade, S.I. Fujita, M. Arai, B.M.
Bhanage, Microwave-assisted additive free synthesis
of nanocrystalline zinc oxide,Powder Technology 203
(2010) 415–418.
7.
K.D. Bhatte, D.N. Sawant, R.A. Watile, B.M.
Bhanage, A rapid, one step microwave assisted
synthesis of nanosize zinc oxide, Materials Letters 69
(2012) 66–68.
8.
F.K. Liu, Y.C. Chang, F.H. Ko, T.C. Chu, Microwave
rapid heating for the synthesis of gold
nanorods,Materials Letters58 (2004) 373–377.
9.
K.D. Bhatte, D.N. Sawant, K.M. Deshmukh, B.M.
Bhanage, Additive free microwave assisted synthesis
of nanocrystalline Mg(OH)
2
and MgO, Particuology
Materials Letters 10 (2012) 384–387.
10.
F.K. Liu, P. X.L. Hu, J.M. Gong, L.Z. Zhang, J.C.
Yu, Continuous size tuning of monodisperse ZnO
colloidal nanocrystal clusters by a microwave-polyol
process and their application for humidity sensing,
Adv. Mater. 20 (2008) 4845–4850.
11.
G.C. Xi, Y.T. He, Q. Zhang, H.Q. Xiao, X. Wang, C.
Wang, Synthesis of crystalline microporous SnO
2
via
a surfactant-assisted microwave heating method: a
general and rapid method for the synthesis of metal
oxide nanostructures, J. Phys. Chem. C 112 (2008)
11645–11649
12.
QingweiZhu,YiheZhang,JiajunWang,FengshanZhou,P
aul K.Chu, Microwave Synthesis of Cuprous Oxide
Micro-/Nanocrystals with Different Morphologies and
Photocatalytic Activities, Journal of Materials Science
& Technology27(2011) 289-295;
13.
Manohar A. Bhosale, Kushal D. Bhatte, Bhalchandra
M. Bhanage, A rapid, one pot microwave assisted
synthesis of nanosize cuprous oxide, Powder
Technology 235 (2013) 516–519;
14.
E. Lu evano-Hipolito, L.M. Torres-Martı´nez, D.
Sanchez-Martı´nez, M.R. Alfaro Cruz, Cu2O
precipitation-assisted with ultrasound and microwave
radiation for photocatalytic hydrogen production,
International Journal of Hydrogen Energy
42(2017)12997-13010
15.
Jun Liu, Shaozhen Wang, Qian Wang, Baoyou Geng,
Microwave chemical route to self-assembled quasi-
spherical Cu2O microarchitectures and their gas-
sensing properties, Sensors and Actuators B 143 (2009)
253–260;
16.
P. Chen, W. Li, T.L. Zhou, Y.P. Jin, M.Y. Gu, J.
Photochem. Photobiol. A: Chem. 168 (2004) 97
17.
Xiaoyan Zhou, Jingjing Shi, Ya Liu, Qingmei Su, Jun
Zhang, Gaohui Du, Microwave-assisted synthesis of
hollow CuO–Cu2O nanosphere/graphene composite as
anode for lithium-ion battery, Journal of Alloys and
Compounds 615 (2014) 390–394
18.
W. Wang, Z. Liao, Y. Wang, X. Wu, F. Qu, X. Zang,
Hydrothermal synthesis of highly symmetric 26-facet
Cu
2
O polyhedral, Crystal Research and Technology
46 (2011) 300–304
