
Expression of Caspase-3 in the Liver and Spleen Rattus norvegicus
Which Infects Bacteria Klebsiella pneumonia and Klebsiella
pneumoniae Extended Spectrum Beta Lactamase
I Gede Andika Sukarya
1
, Willy Sandhika.
1
and Agung Dwi Wahyu Widodo
2
1
Department of Immunology Postgraduate School Universitas Airlangga, Surabaya, East Java, Indonesia
1
Department of Anatomy Pathology, Faculty of Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia
2
Department of Microbiology Clinic, Faculty of Medicine, Dr. Soetomo Hospital, Indonesia
Keywords: Apoptosis, Caspase-3, Klebsiella pneumoniae, Klebsiella pneumoniae ESBL.
Abstract: Klebsiella pneumoniae (K. pneumoniae) is found in nosocomial infections and Gram-negative number two
is the most dangerous. The bacteria K. pneumoniae causes unusual nosocomial infections in hospital
settings. Drug resistance in bacteria K. pneumoniae ESBL production may increase the risk of death at the
time of treatment. Caspase-3 is the hallmark of apoptosis. It can be used as an indicator of virulence of the
infection of K. pneumoniae. This research aims to look at the effects of infection of K. pneumoniae and K.
pneumoniae ESBL against eksprsi caspase-3 in the liver and spleen of rats Rattus novergicus. The study
uses 12 healthy rats that were divided into three groups, namely the control group, infection K. pneumoniae,
and infection K. pneumoniae ESBL. Each group of samples taken from the liver and spleen organs after 24
hours of the infection process and immunohistochemical expression to see caspase-3. Of the research results
obtained, the percentage of caspase-3 expression in the liver and spleen organs in rats that infect bacteria K.
pneumoniae, are higher than K. penumoniae ESBL. The expression of caspase-3 in the liver and spleen
organs is steeper than the infection of K. pneumoniae. We conclude that the bacteria K. pneumoniae is more
virulent than K. pneumoniae ESBL.
1 INTRODUCTION
Klebsiella pneumoniae (K. pneumoniae) is found
in nosocomial infections and Gram-negative number
two is the most dangerous (Tsai et al., 2009). The
bacteria K. pneumoniae causes unusual nosocomial
infections in hospitals (Woldu, 2016). The bacteria
K. pneumoniae is very dangerous for patients with
immunocompromised and immunodeficiency,
causing damage to organs (Wu, 2015). The
transmission of K. pneumoniae in hospitals has
become a particular concern in Europe, the United
States, Argentina, and Australia. Infections caused
by K. pneumoniae result in longer treatment times in
hospital and resistance to antibiotics (Brisse et al.,
2006).
Extended Spectrum Beta Lactamase (ESBL) K.
pneumoniae produces an aggravating risk factor for
infection. The bacteria K. pneumoniae ESBL
production is resistant to beta-lactam antibiotics and
are at risk of adding to long treatment times in ICUs
(Toner et al., 2016). The virulence of K. pneumoniae
this decade can infect normal or healthy individuals,
due to resistance to drugs and hipervirulen (Paczosa,
2016)
Apoptosis plays a role in bacterial infection.
Apoptosis affects the immune cells that are very
important in the course of the infection. Regulation
of apoptosis is an important aspect of the host cell
response to stress and infection and should be
continual. Caspase in the apoptosis process plays a
role in cellular processes, which occur before
apoptosis happens (Silva, 2009). Apoptosis triggered
by the TNFα is produced by inflammation from the
bacterial infection Klebsiella pneumuniae good
through the intrinsic or extrinsic pathways triggered
by various cellular responses through the endotoxin
lipopolysaccharide (LPS). The Endotoxin bacteria K.
pneumuniae often triggers endothel cell damage so
multiple organ failure can occurs. Endotoxin triggers
the inflammatory process, which is sustainable and
increases the incidence of cell death in the follicle
cell dendritic, dendrite cells, neutrophils, CD4
+
,
244
Andika Sukarya, I., Sandhika, W. and Wahyu Widodo, A.
Expression of Caspase-3 in the Liver and Spleen Rattus norvegicus Which Infects Bacteria Klebsiella pneumonia and Klebsiella pneumoniae Extended Spectrum Beta Lactamase.
DOI: 10.5220/0007540702440248
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 244-248
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

CD8
+
, and B cells (Paczosa, 2016). Caspase-3 is a
death protease activation of mediators that
frequently programs cell death (apoptosis), and
catalyzes the cleavage of cell specific proteins of
some. Caspase-3 is the hallmark of apoptosis, and
indispensable for the condensation of the cell
apoptosis and fragmentation of DNA. Caspase-3 is
very important in the process of apoptosis (Porter,
1999).
The spleen immune system is responsible for
protecting the body from the invasion of pathogens
and detecting old cells, damaged mechanically, and
distorted, which can lead to the formation of tumors.
The spleen plays out a double simultaneous reaction
against bacterial antigens and allogenes. As a
secondary lymphatic system, due to the number of
lymphocytes in the spleen area, more and bacteria
will be taken towards the organ spleen.
Lymphocytes in the spleen undergo apoptosis. The
liver is the largest location of kupffer cells, NK cells,
and NKT cells. Cells, NK cells and kupffer NKT
cells are activated by the stimulation of APC, both
directly and indirectly, to respond to bacterial
infections. Activation of the cells that are in the liver
apoptosis are triggered to respond to infections
(Wang, 2014). The expression of caspase-3 denotes
prosen apoptosis, which occurs during the ongoing
infection process (Porter, 1999).
In this study, we will present the expression of
caspase-3 in infection K. pneumuniae and K.
pneumuniae production of ESBL in Rattus
norvegicus. Expression of caspase-3 in the liver and
spleen of a rat show virulence infection K.
pneumuniae and K. pneumuniae ESBL. This can be
seen with the virulence of the infection K.
pneumuniae and K. pneumuniae in the liver and
spleen organs.
2 MATERIALS AND METHODS
2.1 Animals
The rat Rattus novergicus; male rats that are healthy
and not exposed to infection. They are characterized
by the movement of agile rats, aged three months,
with a weight of 200–250 grams.
2.2 Bacteria
The bacteria K. pneumoniae ESBL and K.
pneumoniae derived from Installations Clinical
Microbiology of Dr. Sutomo Surabaya. Made with
10
5
CFU of bacteria concentrations of Phosphate-
buffered saline (PBS).
2.3 Method
The healthy rat is selected by raffled and is given a
mark or code of any group consisting of four rats.
Injection materials on the peritoneum in Quadrant 3
included as much as 1ml (Group control in injection
PZ (Phisiological zouth), a group of K. penumoniae
injection on concentrations with 10
5
CFU K.
pneumoniae, and groups of K. pneumonie ESBL
injection on concentrations with 10
5
CFU K.
penumonie ESBL) in each group of animals. The
rats were observed for 24 hours. The livers and
spleen organs were retrieved 24 hours after surgery.
The rat’s liver and spleen fixation buffer into the
tissue preparation and formaldehyde (formalin fixed
and paraffin embedded section). The sample was
made of paraffin blocks and cutting samples with a
thickness of 4µm, using either a microtome or tool
placed on the microscope slide. An immunostaining
process was performed using the
immunohistochemistry reagents Caspase-3 (Bioss
Antibodies, Bioss, USA).
2.4 Immunohistochemistry
Deparaffinization and rehydration; Wash slides
twice in Xylene for three minutes each time in real
time; Wash slides in Xylene 1:1 with 100% ethanol
for three minutes in real time; Wash slides twice in
100% ethanol for three minutes each in real time;
Wash slides twice in 95% ethanol for three minutes
each in real time; Wash slides in 70% ethanol for
three minutes in real time; Wash slides in 50%
ethanol for three minutes in real time; Rinse slides
gently with running distilled water for five minutes
in real time. Antigen retrieval; Boil slides in 0.01M
sodium citrate buffer (pH6) at 100°C for 15–20
minutes; Remove the slides from the heat and allow
them to stand at real time in the buffer for 20
minutes; Rinse twice with TBST for five minutes in
real time. Immunostaining; block with endogenous
peroxidase with 3% hydrogen peroxide for 30
minutes; block with 5% serum or BSA for two hours
in real time; drain blocking buffer from slide;
incubate slides with the diluted primary antibody
overnight at 4°C with gentle agitation; Wash slides
twice with TBST for five minutes in real time;
incubate slides with diluted conjugated secondary
antibody for two hours in real time with gentle
agitation; wash slides twice with TBST for five
minutes in real time; develop with chromogen for 10
Expression of Caspase-3 in the Liver and Spleen Rattus norvegicus Which Infects Bacteria Klebsiella pneumonia and Klebsiella
pneumoniae Extended Spectrum Beta Lactamase
245
minutes in real time; wash slides in distilled water
for one minute in real time; counterstain (if
required); Dehydrate when using a chromogen
substrate that is alcohol insoluble by washing slides
in 80%, 95%, 100%, and Xylene each for one
minute in real time; Mount coverslips; check out the
slides and calculate the percentage of positive cells
caspase-3 there are liver and lymphatic organs.
3 RESULT
The research results were obtained as a percentage
of the number of cells that undergo caspase-3 on the
organ spleen and liver in the treatment group
infection K. pneumoniae, K. pneumoniae ESBL and
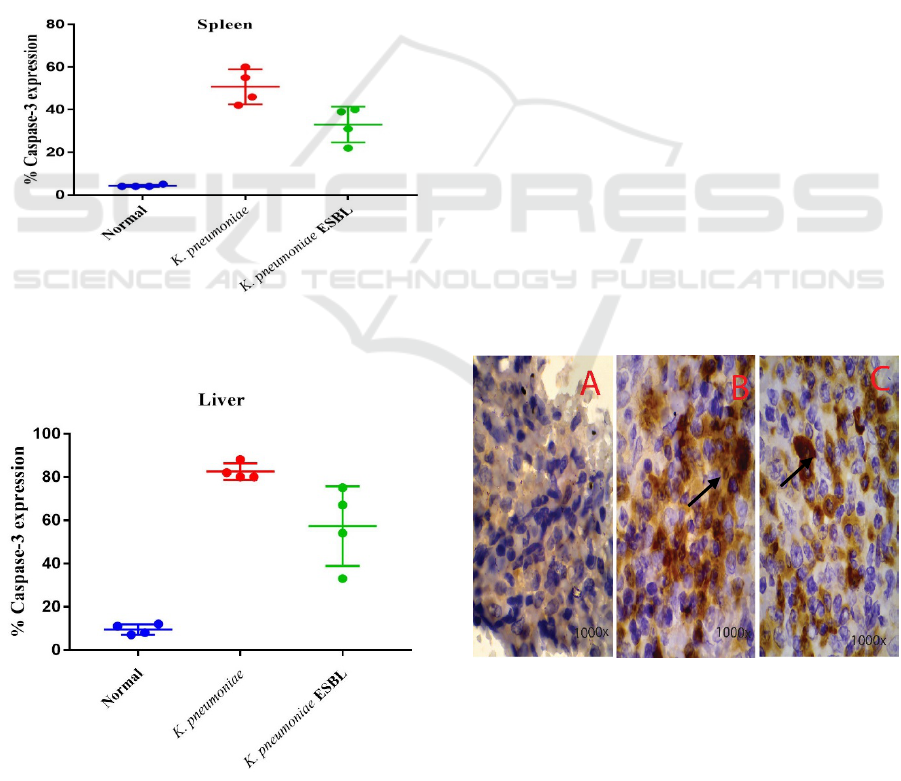
the control group (Figures 1 and 2).
Figure 1: Box-plots the percentage of cells that undergo
caspase-3 in the spleen.
Figure 2: Box-plots the percentage of cells that undergo
caspase-3 in the liver.
Figure 1 shows the percentage of caspase-3 in
the lymphatic organs. The group treatment in
infection K. pneumoniae are higher than group
treatment on infection of K. pneumoniae ESBL and
those in the control group. The value of the median
is the highest to the lowest in succession, i.e. the
Group of K. pneumoniae (45.5), K. pneumonia
ESBL (35), and the control group (4).
Figure 2 shows the percentage of caspase-3 in
the liver organ on group infection K. pneumoniae in
treatment was higher in the treatment group of
infection K. pneumoniae ESBL and those in the
control group. The value of the median is the highest
to lowest in succession i.e., the Group of K.
pneumoniae (81), K. pneumonia ESBL (60.5) and
the control group (9).
Figure 3 shows R. norvegicus spleen cells
demonstrating the immunostaining Caspase-3; The
control group is shown in picture A with a
magnification microscope 1000x; K. pneumoniae
group is shown in picture B with a magnification
microscope 1000x. There are many cells that express
caspase-3, such as arrows. Labelled cells in the
spleen are experiencing the process of apoptosis, and
groups of K. pneumonia ESBL in picture C shows
the cells at 1000x magnification. On the organ in a
bacterial infection K. pneumoniae and K.
pneumoniae ESBL shows the expression of caspase-
3 on the cell. This marks the process of apoptosis
occurring on the spleen, caused by a bacterial
infection K. pneumoniae, causing almost 60% of
damage to the spleen organ.
Figure 3: Cells in the spleen tissues of R. norvegicus in
immunostaining caspase-3 in the control group Figure A,
K. pneumoniae Group Figure B and K. pneumonia ESBL
Figure C.
ICPS 2018 - 2nd International Conference Postgraduate School
246
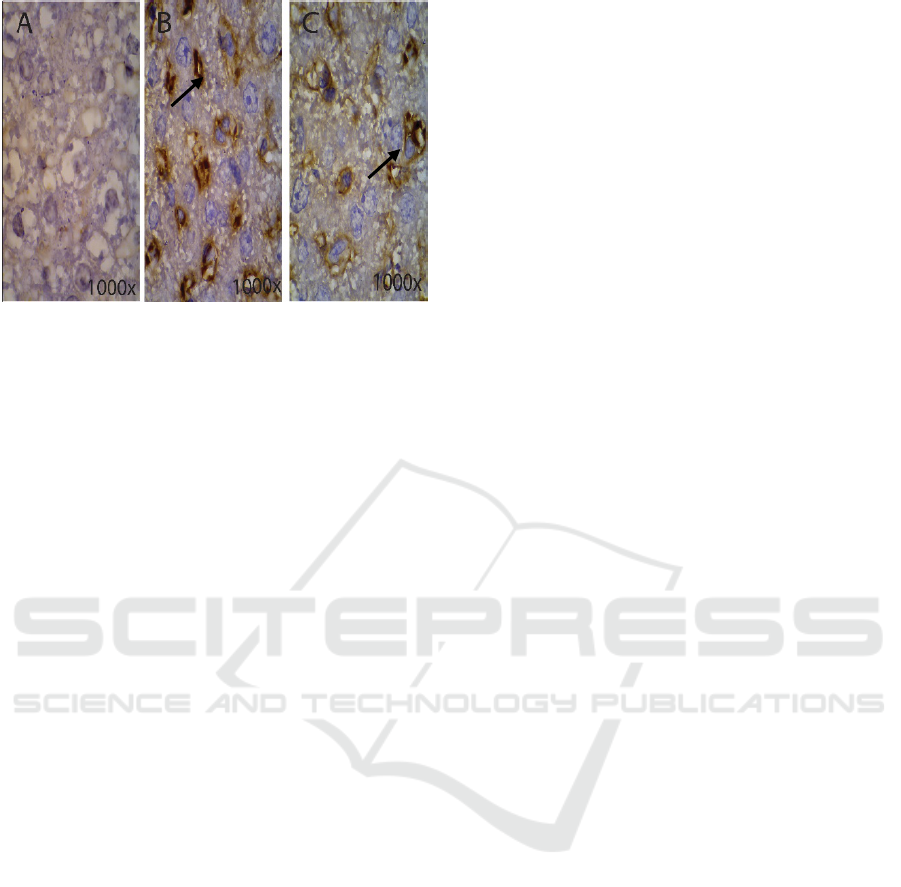
Figure 4: Cells on R. norvegicus in liver tissue
immunostaining caspase-3 in the control group Figure A, ,
K. pneumoniae group Figure B and K. pneumoniae ESBL
group Figure C.
Figure 4 shows R. norvegicus demonstrating the
liver tissue in immunostaining caspase-3; the control
group is shown in picture A at 1000x magnification
using a microscope; the K. pneumoniae group is
shown in picture B at 1000x magnification using a
microscope. There are many cells that express
caspase-3, such as arrows and labelled cells in the
liver that are experiencing the process of apoptosis.
For the K. pneumonia ESBL group, picture C shows
cells at 1000x magnification using a microscope.
On the organ in a bacterial infection, K. pneumoniae
and K. pneumoniae ESBL shows the expression of
caspase-3 on the cell. This marks the process of
apoptosis occurring in liver organs caused by the
bacterial infection K. pneumoniae, causing 80%
liver organ damage.
4 DISCUSSION
There was an increase in the percentage of caspase-3
in liver and spleen organs in a group of animal
models with the infections K. pneumoniae and K.
pneumoniae ESBL. Injection of germs in an animal
model is done through the peritoneal line. The test
compound injected into the peritoneal cavity will be
absorbed into the portal circulation and transported
to the liver (Shayne et al., 2013). The liver receives
the blood vein of the portal and the arterial blood,
liver, and spleen are important components in the
defense against infection entering the blood stream.
To achieve this role, the liver and the spleen contain
many innate and adaptive immune cells specifically
to detect and capture pathogens from the blood
stream. Furthermore, the immune cells participate in
the immune response that leads to the purge of
pathogens, the recruitment of leukocytes and antigen
presentation to lymphocytes in the blood stream. An
increasing number of caspase-3 in liver organs can
be caused due to a high inflammatory process, which
triggers the cells to hepatocyte or innate underwent
apoptosis. There is a balance between activation and
tolerance that characterizes the liver and spleen as
immunological organ front line (Jenne and Kubes,
2013).
The spleen is an organ of the lymphatic system
and inserted into the bloodstream is a collection of
lymphoid tissues. The spleen immune system is
responsible for protecting the body from the
invasion of pathogens and detecting old cells,
damaged mechanically and distorted that can lead to
the formation of tumors. Recent studies prove the
dominant role in the simultaneous double reaction
against bacterial antigens and allergens. The spleen
is the seat of an innate and adaptive immune system.
Microbial network penetration evokes an innate
system direct reaction, while the adaptive immune
response involves the interaction of cells that
recognize specific antigens in the context with the
MHC presented by a cell that gives rise to antigens
(Wluka et al., 2006) A secondary lymphatic system
allows the number of lymphocytes in the spleen area
to increase, and infected bacteria is carried towards
the spleen organ. Lymphocytes in a lien allows will
undergo apoptosis. Of the function that has been
described above, an increasing number of caspase-3
in liver and spleen organ can be caused due to the
high inflammatory process to trigger the cells to
hepatocyte or undergoing innate apoptosis.
Caspase-3 positive cells in the spleen and liver
organs in animal models with the infection of K.
pneumoniae are higher than animal models in
infection of K. pneumoniae ESBL. The bacterium K.
pneumoniae and K. pneumonie ESBL triggered a
wide range of cellular response through the
endotoxin lipopolysaccharide (LPS). The ongoing
process of inflammation triggers endotoxins and
increases the incidence of apoptosis on dendrite
cells, follicular dendritic cells, and neutrophils
nanotechnologies, the number of CD4
+
, CD8
+
and B
cells (Paczosa, 2016). K. pneumoniae protects a
variety of humoral defense mechanisms, such as the
mechanism of bacterial resistance to complement the
damage. In phagocytosis, this did not happen on K.
pneumoniae ESBL. Human beta-defensins 1 (HBD-
1) and HBD-2 inefficient kill K. pneumoniae as
HBD-3. K. pneumoniae ESBL production is more
susceptible to HBD (Moranta et al., 2010). Caspase-
3 indicates that high apoptosis occurrence is very
high and is prominent regarding pathological liver
Expression of Caspase-3 in the Liver and Spleen Rattus norvegicus Which Infects Bacteria Klebsiella pneumonia and Klebsiella
pneumoniae Extended Spectrum Beta Lactamase
247

disease. The end result of caspase-3 is the high cause
of dysfunction of the liver, cirrhosis, and
tumorigenesis (Wang and Lien, 2013).
REFERENCES
Brisse, S., Grimont, F., Grimont, P.A.D., 2006. The
genus Klebsiella. In The Prokaryotes: A Handbook on
the Biology of Bacteria, 3rd edn, vol. 6, pp. 159–196.
Jenne, C.N.,Kubes, P., 2013. ‘Immune Surveillance by the
Liver’ natur immunology, vol.14(10) october 2013.
996-1006. accessed at 6 August 2017,
doi:10.1038/ni.2691
Paczosa, M.K., Mecasas, J., 2016. ‘Klebsiella
pneumoniae: Going on Offense with a Strong
Defense’, Microbiology and Molecular Biology
Review, vol.80:692-661.
Porter, A.G,, Jänicke, R,U,, 1999. ‘Emerging Roles of
Caspase-3 in Apoptosis’, Cell Death and
Differentiaton, vol.6;99-104.
Shon, AS, Bajwa, RPS, Russo, TA 2013, ‘Hypervirulent
(hypermucoviscous) Klebsiella pneumoniae: A New
and Dangerous Breed’, Virulence, vol.4(2). 107-118.
Silva, F.P., Nizet, V., 2009. ‘Cell Death During Sepsis:
Integrating of Disintegration in the Inflammatory
response to overwhelming Infection’, Cell Death and
Disease, vol.14:509-521, accessed at 6 August 2017.
Doi 10.1007/s10495-009-0320-3
Toner, L., Papa, N., Aliyu, H.S., Dev, H., Lawrentschuk,
N., Al-hayek, S. 2016. ‘Extended-spectrum
beta-lactamase-producing Enterobacteriaceae in
hospital urinary tract infections: incidence and
antibiotic susceptibility profle over 9 years’, World
Journal Urology, 34:1031-1037.
Tsai, S.S., Huang, C.J., Chen, T.S., Sun, H.J., Wang, C.C.,
Lin, F.S., Hsu, S.R.B., Lin D.J., Huang, Y.S., Huang,
Y.Y., 2010. ‘Characteristics of Klebsiella pneumoniae
Bacteremia in Community-acquired and Nosocomial
Infections in Diabetic Patiens’. Chang Gung Medical
Journal, Vol.33 No.5: 532-539.
Wang, K., Lin, B., 2013, ’Review article
pathophysiological of hepatic apoptosis’ ISRN
Hepatology, Hindawi Publishing Corporation,
vol.2013, accessed at 6 August 2017,
http://dx.doi.org/10.1155/2013/740149.
Wluka, A., Olszewski, W.L., 2006. ‘Innate and adaptive
processes in the spleen’ Ann Transplant, vol.11:22-9
Woldu, A.M., 2016. ‘Klebsiella pneumoniae and Its
Growing Concern in Healthcare Settings’ Clinical &
Experimental Pharmacology, 6(1):199.
Wu, M., Li, X., 2015. ‘Klebsiella pneumoniae and
Pseudomonas aeruginosa. Molecular Medical
Microbiology’ Research gate.
Elsevier. Ch.87:1547-
1564, accessed at 6 August 2017, doi:http//
dx.doi.org/10.1016/B979-0-12-397169-2.00087-1.
ICPS 2018 - 2nd International Conference Postgraduate School
248
