
Differences of Caspase-3 Expression in the Spleen and Liver of Sepsis
Models in Rats Infected with Escherichia coli ESBL and Klebsiella
pneumoniae Carbapenemase
Lisa Savitri
1
, Willy Sandhika
2
, and Agung Dwi Wahyu Widodo
3
1
Department of Immunology, Postgraduate School, Universitas Airlannga, Surabaya, East Java, Indonesia
2
Department of Pathology, Faculty of Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia
3
Department of Microbiology Clinic, Faculty of Medicine, RSUD Dr. Soetomo, Surabaya, East Java, Indonesia
Keywords : Caspase-3 expression, Escherichia coli ESBL, Klebsiella pneumoniae carbapenemase
Abstract : Sepsis is the leading cause of death in the world. Sepsis patients with Extended Spectrum β-lactamase
(ESBL)-producing bacterial infections were 57.4% Escherichia coli, 21.35% Enterobacter sp, and 21.3%
Klebsiella sp. Caspase-3 is the most important caspase effector responsible for morphological and biological
changes in apoptotic cells. This type of research is true experimental with a post-test only control group
design, using one control rat group and two groups of rats infected with E. coli Extended Spectrum β-
lactamase (ESBL) and Klebsiela pneumoniae carbapenemase (KPC) for 24 hours to find out the different
expression of caspase-3 in the spleen and liver of those infected rats. Expression of caspase-3 was observed
by staining the spleen and liver with caspase-3 p12 subunit antibody. Cells expressing caspase-3 were
counted under the light microscope. The results showed that caspase-3 expression in the KPC infected
spleen group was 65.25±12.69%, whereas E. coli ESBL was 33.75±3.862%. This is thought to be
influenced by the presence of antigen differences between the two bacteria, thus the possibility of apoptosis
in lymphocyte cells caused by KPC would be higher when compared with those infected with E. coli ESBL.
Caspase-3 liver expression in the KPC group had a value of 58.75±4.031%, while the E. coli ESBL infected
was 48.75±6.292%. It may be affected by differences in soluble factors of both bacteria, thus the possibility
of apoptosis in hepatocyte-induced cells by KPC will be higher when compared with those that are E. coli
ESBL infected.
1 INTRODUCTION
Sepsis is a clinical syndrome that occurs due to
excessive body response to stimulation of
microorganism products (Guntur, 2007). Sepsis is
the leading cause of death in the world and the cause
of deaths in Intensive Care Units (ICU). It is
estimated that about 1,400 patients die in the ICU
because of sepsis (Poeze et al., 2004). Apoptosis is
commonly involved in bacterial infections and
pathogenesis. During bacterial infections, virulent
factors (mostly endotoxins) are produced and
secreted from pathogens and trigger apoptotic
signals. Research on caspase-3 is important, as it is
the most important caspase effect responsible for
morphology and biological changes seen in
apoptotic cells (Ghatage et al., 2013). Based on this
phenomenon, it is necessary to conduct research to
determine the increase of expression caspase-3 on
the spleens and livers of rats infected with
Escherichia coli Extended Spectrum β-lactamase
(ESBL) and Klebsiela pneumoniae carbapenemase
(KPC).
2 METHODS
2.1 Type and Design of Research
The type of this research is pure laboratory (true
experimental) research using post-test only for the
control group (data retrieval done after treatment)
and compared with the control group.
Savitri, L., Sandhika, W. and Wahyu Widodo, A.
Differences of Caspase-3 Expression in the Spleen and Liver of Sepsis Models in Rats Infected with Escherichia coli ESBL and Klebsiella pneumoniae Carbapenemase.
DOI: 10.5220/0007540802490252
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 249-252
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
249
2.2 Place and Time of Research
2.2.1 Research Place
This study was conducted in several locations: the
Animal Unit Laboratory of Biochemistry, Faculty of
Medicine, Universitas Airlangga, Microbiology
Laboratory of RSUD Dr. Soetomo, Surabaya, and
Anatomical Pathology Laboratory, Faculty of
Medicine, Universitas Airlangga.
2.2.2 Research Time
This study was conducted for approximately three
months, from October 2017 to December 2017.
2.3 Research Objects
The object of the research used in this research was
rats (Rattus norvegicus); a male strain Wistar aged
about eight to 12 weeks with a body weight of 150–
200 grams that came from the animal unit’s
biochemistry laboratory, Faculty of Medicine,
Universitas Airlangga.
2.4 Animal Treatment
Adapted rats were injected in the peritoneum section
with the following treatments: 1) group one as
normal control, i.e. injected aqua pro-injection-free
pyrogen; 2) group two as treatment one, i.e. injected
rat E. coli ESBL with dose 1x10
5
CFU/ml; and 3)
group three as treatment two, i.e. rat injected KPC
with dose 1x10
5
CFU/ml. After 24 hours post-
exposure of polymicrobial sepsis animals will show
apoptosis in the spleen and liver.
2.4.1 Caspase-3 Expression Observation on
the Spleen and Liver of Rats
Observations of the caspase-3 expression on the
liver and spleen of rats were performed by painting a
primary antibody caspase-3 p12 subunit antibody
(host: rabbit, target protein: caspase-3 p12 subunit,
clonality: polyclonal, isotype: IgG, entrez gene: 836,
source: KLH conjugated synthetic peptide derived
from human caspase-3 p12 subunit, purification:
purified by protein A) Bioss Antibodies production.
The caspase-3 expression was observed using the
immunohistochemical method. Caspase-3 expressed
when exposed to the brown color in cytoplasmic
sections, but if in clear cytoplasmic sections, it can
be stated that caspase-3 is unexpressed. The
calculation of caspase-3 expression is done by
calculating the cell expressing caspase-3 divided by
all the preserved cells, then multiplying by 100%, so
the data is expressed using a percentage.
3 RESULT
3.1 Caspase-3 Expression in Spleen of
Rats with E. coli ESBL and KPC
Table 1 : Average Data and Standard Deviation Caspase-3
Expression in a Rat’s Spleen with Control Treatment, E.
coli ESBL Infection, and KPC Infection (%).
Group x±SD Median Max-Min
Control
(n=4)
4,25±0,5 4 5–4
E. coli
ESBL
(
n=4
)
33,75±3,86
2
33,5 38–30
KPC (n=4)
65,25±12,6
9
67 78–49
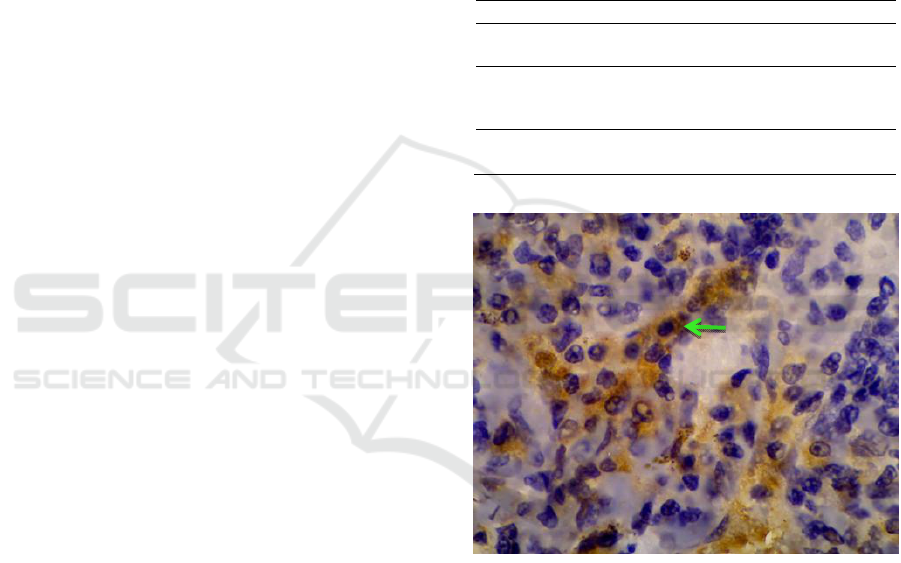
Figure 1 : Expression of caspase-3 in spleen of rats
infected with E. coli ESBL are stained brown (green
arrow). Magnification: x1000.
ICPS 2018 - 2nd International Conference Postgraduate School
250
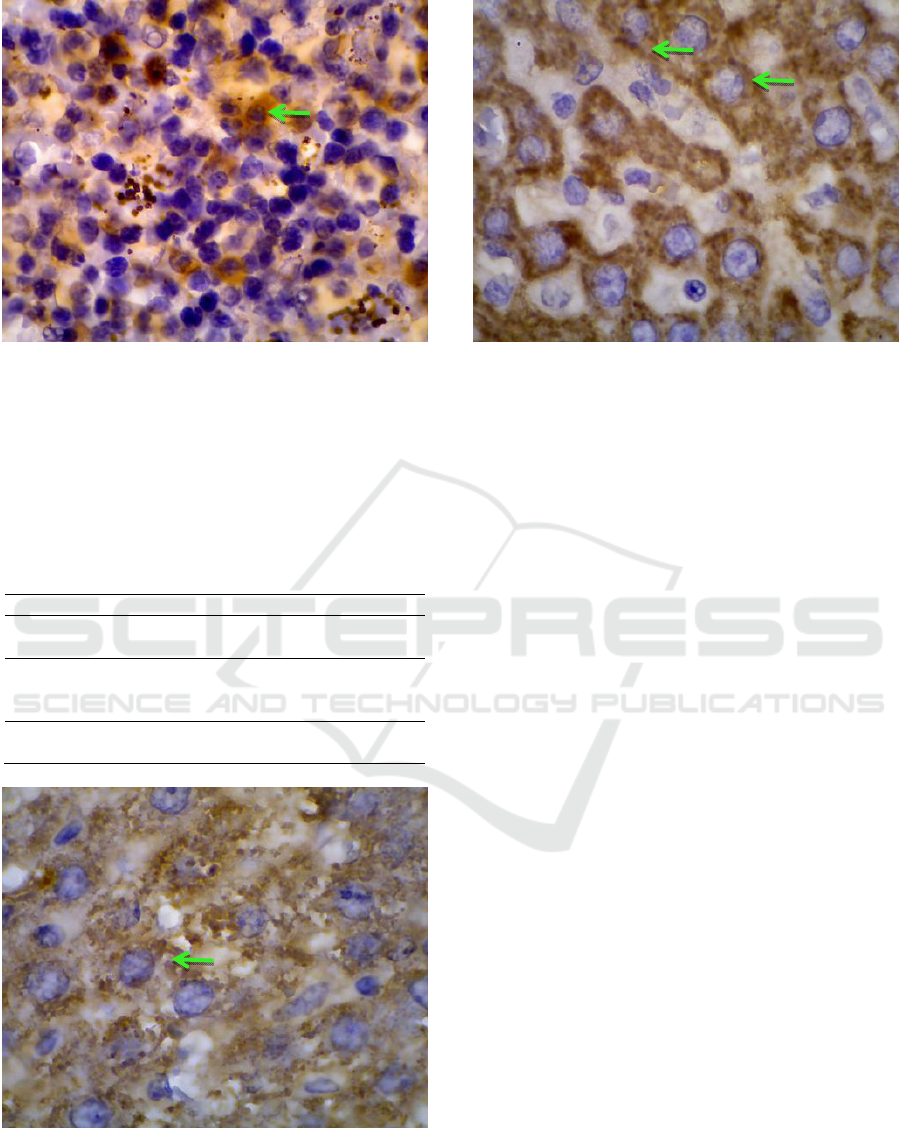
Figure 2 : Expression of caspase3 in spleen of rats
infected with KPC are stained brown (green arrow).
Magnification: x1000.
3.2 Caspase-3 Expression in the Livers
of Rats with E. coli ESBL and KPC
Table 3.2 Average Data and Standard Deviation Caspase
3 Expression on Rat’s Liver with Control Treatment, E.
coli ESBL Infection, and KPC Infection (%).
Group x±SD Median Max-Min
Control
(n=4)
9,5±2,38 9,5 12–7
E. coli
ESBL
(n=4)
48,75±6,29
2
46,5 58–44
KPC (n=4)
58,75±4,03
1
59 63–54
Figure 3 : Expression of caspase-3 in livers of rats
infected with Escherichia coli ESBL are stained brown
(green arrow). Magnification: x1000.
Figure 4: Expression of caspase-3 in the livers of rats
infected with K. pneumoniae carbapenemase are stained
brown (green arrow). Magnification: x1000.
4 DISCUSSION
4.1 Caspase-3 Expression in the Spleen
of Rats with E. coli ESBL and KPC
Increased caspase-3 expression in rat-infected KPC
was higher when compared with those infected with
E. coli ESBL. It can be influenced because of
differences in antigens possessed by KPC with E.
coli ESBL. The KPC capsule consists of an O
antigen which is a liposaccharide consisting of a
repeating polysaccharide unit. Antigen O is resistant
to heat and alcohol. The second antigen is the K
antigen. The K antigen is outside the antigen O and
is a capsular polysacharida. The K antigen may
interfere with agglutination through antiserum O and
is associated with virulence. Both of these antigens
increase the pathogenity of KPC.
During bacterial infections, virulent factors are
produced and secreted by pathogens and trigger
apoptotic signals. In general, cells undergo apoptosis
of two main pathways, extrinsic pathways (dead
receptor pathways) and intrinsic pathways
(mitochondrial pathways) (Jin and El-Deiry, 2005;
Ayala et al., 2007). After releasing specific pro-
apoptotic proteins, such as cytochrome c,
smac/DIABLO, AIF, and Endo G, the execution
path begins with caspase activation 3. Its main
purpose is to bind and activate the caspase
recruitment domain (CARD), Apaf-1, and procaspse
9, which leads to the formation of apoptosome.
Next, it leads to activation of caspase-9 and further
Differences of Caspase-3 Expression in the Spleen and Liver of Sepsis Models in Rats Infected with Escherichia coli ESBL and Klebsiella
pneumoniae Carbapenemase
251

activates the caspase-3 effector, which completes the
apoptotic pathway. These are apoptosome
formations and activation of the caspase effectors
that cause apoptotic events, such as chromatin
condensation, plasma membrane asymmetry, and
cellular blebbing (Abud, 2004; Nikitakis et al.,
2004).
4.2 Caspase 3 Expression in the Livers
of Rats with E. coli ESBL and KPC
Based on previous research, it was found that there
was an increase of caspase-3 expression in the livers
of the of rats in the group infected with E. coli ESBL
and infected by KPC. Injection of bacteria in the rats
was done through an intraperitone injection
pathway. Bacteria injected into the peritoneum
cavity will be absorbed into the portal circulation
and transported to the liver. As an organ that acts as
a recipient of portal and arterial blood vessels, the
liver is an important component in the defense
against blood-borne infections.
Increased caspase-3 expression in rats
infected with KPC was higher when compared with
E. coli ESBL-infected rats. This might be affected
by differences in soluble factors of bacteria that can
induce host cells. Factors involved in the virulence
of KPC strains include capsular serotypes,
lipopolysaccharides, ironscavenging systems,
fimbrial and non-fimbrial adhesions. The
polysaccharide capsule surrounding KPC protects
itself against the action of phagocytosis and serum
bactericidal and may be considered the most
important determinant of the virulence of KPC.
Liver damage is associated with the incidence of
liver cell apoptosis (Mordue et al., 2001). Apoptosis
through intrinsic pathways in liver cells is caused by
a soluble factor of bacteria that can induce host cells,
and is thus toxic to other cells. This soluble factor
causes the mitochondria to release ROS. These
bacterial infections cause the mitochondria to
produce ROS and trigger the release of cytochrome
c (Nomura et al., 2000). Cytochrome c will trigger
caspase-9 to bind to the caspase effect, i.e. caspase-
3, resulting in apoptosis (Yoon et al., 2002).
5 CONCLUSIONS
The increase in the caspase-3 expression in the
spleens of rats infected with KPC was higher
compared with that of rats infected with E. coli
ESBL. The different antigens in those two different
bacteria may have contributed to the expression of
caspase-3 and the possibility of apoptosis in the
lymphocyte cells caused by KPC would be higher
when compared with those infected with E. coli
ESBL. Similarly, the different expression of
caspase-3 in the liver of rats, infected with those two
bacteria, may be caused by the soluble factors
secreted by both bacteria.
REFERENCES
Abud HE. 2004. Shaping Developing Tissues by
Apoptosis. Cell Death Differ, 11: 3155-62.
Ghatage DD, Gosavi SR, Ganvir SM, and Hazarey
VK. 2013. Apoptosis: Molecular Mechanism.
Journal of Orofacial Sciences., 4: 103-107.
Green DR and Reed JC. 1998. Mitochondria and
Apoptosis. Science 281:1309-12.
Guntur AH. 2007. Imunopatobiologik sepsis dan
penatalaksanaanya. Simposium Nasional SEPSIS
dan Antimikrobial Terkini. Surakarta: PETRI, pp: 31-
6.
Jin and El-Deiry, W. S. 2005. Stabilization of p53 by CP-
31398 inhibits ubiquitination without altering
phosphorylation at serine 15 or 20 or MDM2
binding. Mol. Cell. Biol. 23, 2171- 2181.
Mordue, D.G., F. Monroy., M.L. Regina.,C.A. Dinarello
and L.D. Sibley. 2001. Acute Toxoplasmosis Leads to
Lethal Overproduction of Th1 Cytokines. The
American Association of Immunologists, 167:4574-
4584.
Nikitakis NG, Sauk JJ, and Papanicolaou SI. 2004. The
Role of Apoptosis in Oral Disease: Mechanisms;
Aberrations in Neoplastic, Autoimmune, Infectious,
Hematologic, and Developmental Diseases; and
Therapeutic Opportunities. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod., 97: 476-490.
Nomura, K., H. Imai, T. Koumura, T.Koebayashi and
Y. Nakagawa. 2000. Mithochondrial hospholipid
hydroperoxide glutathione peroxidase inhibists the
release of cytocrome c from mithichondrial by
suppressing the peroxidation of cardiolipin in
hypoglycaemia induced apoptosis. Biochem J., 351:
183-193.
Poeze M, Ramsay G, Gerlach H, Rubulotta F, Levy M.
2004. An international sepsis survey: a study of
doctors' knowledge and perception about sepsis.
Critical Care. 8(6): 409-13.
Yoon, J.H. and G.J. Gores. 2002. Death Receptor-
mediated apoptosis and the liver. J. Hepatology. 37:
400-410.
ICPS 2018 - 2nd International Conference Postgraduate School
252
