
Differences of Caspase-3 Expression in Liver and Spleen of Rattus
norvegicus Infected with Streptococcus pyogenes and Acinetobacter
baumannii
Nurul Amalia
1
, Agung Dwi Wahyu Widodo
2
, and Willy Sandhika
3
1
Departement of Immunology Postgraduate School Airlangga University Surabaya, East Java, Indonesia
2
Departement of Microbiology Clinic, Dr. Soetomo Hospital Surabaya, East Java Indonesia
3
Departement of Anatomy Pathology, Faculty of Medicine, Universitas Airlangga, Surabaya, Jawa Timur, Indonesia
Keywords: Apoptosis, Caspase-3, Streptococcus pyogenes, Acinetobacter baumannii.
Abstract: Streptococcus pyogenes as exotoxin-producing bacteria are the most common cause of pharyngitis infection.
Acinetobacter baumannii as endotoxin-producing bacteria are found in nosocomial infections. The
infections that has already spread to all organs with decreased immune system undergo apoptosis in liver
and spleen. Caspase-3 is a death protease most commonly activated by apoptotic mediator. The higher the
caspase-3 expression, the higher the severity of the disease, which can cause the organ to experience
dysfunction or failure. This study was aimed to observe the expression of caspase-3 in the liver and spleen
of Rattus norvegicus, infected by Streptococcus pyogenes and Acinetobacter baumannii. This is a true
experimental with a post-test only control-group design. The healthy rats were randomly selected and
injected with 1ml (PZ, suspension of bacteri A. baumannii and S. pyogenes) in the peritoneum in quadrant 3
for each group of experimental animals and observed for 24 hours. After 24 hours, surgery took place for
the removal of the liver and spleen. Organ tissues where fixed into a formalin buffer and tissue was prepared
for IHC caspase-3.The results showed that the mortality rate of Rattus norvegicus infected by A. baumannii
was higher than those infected by S. pyogenes. Caspase-3 expression in the liver of A. baumannii group was
48, S. pyogenes was 22.5, and the control group was 9.5. The mean value of the caspase-3 index in the
spleen of the A. baumannii group was 28.5, S. pyogenes was 17, and the control group was 4.
1 INTRODUCTION
The infection is caused by bacteria that produces
toxins and endotoxins. In general, infectious
diseases are caused by bacteria, fungi, viruses, and
parasites. Streptococcus pyogenes as toxin-
producing bacteria is the most common cause of
infectious pharyngitis. S. pyogenes bacteria is
included in Group A streptococci of serology (Group
A Streptococcus, GAS) (Fidler et al., 2015). S.
pyogenes bacteria can infect the host body's defenses
when dropped or when the organism can penetrate
past the host's defense (Ramachandran, 2017).
The bacteria Acinetobacter baumannii is an
endotoxin- producing bacteria found in nosocomial
infections and harmful gram-negative bacteria
(Bigot and Suzana, 2017). In recent years the
bacteria A. baumannii has increased over the
nosocomial infections in humans. The bacteria A.
baumannii is found as a nosocomial infection-
causing bacteria in the urinary tract, an infection of a
wound, and in vascular surgery, particularly in
patients with low immune systems located in the
ICU. Research in Indonesia has determined the
bacteria Acinetobacter as one gram-negative that
most often infected are 25.8% (Norhamdani, 2004).
Apoptosis has an important role in bacterial
infections of S. pyogens and A. baumannii.
Apoptosis affects the immune cells,which are very
important in the course of an infection. Apoptosis
can be determined not only on a certain type of
bacteria, but in bacterial infections in a variety of
species. The regulation of apoptosis is an important
aspect of the host cell response against stress,
infection, and must be controlled (Ulett and
Elisabeth, 2006).
A variety of stimuli can trigger apoptosis from
within or outside the cell, for example, infection
with microorganisms, cell cycle, or signaling the
258
Amalia, N., Dwi Wahyu Widodo, A. and Sandhika, W.
Differences of Caspase-3 Expression in Liver and Spleen of Rattus norvegicus Infected with Streptococcus pyogenes and Acinetobacter baumannii.
DOI: 10.5220/0007541002580262
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 258-262
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All r ights reserved

death of cell-surface receptors, development, DNA
damage and occurrence of inflammation.
Inflammatory Cytokines
(TNF) can constantly
induce activation of caspase-8,
caspase-3, and the
fragmentation of DNA through membrane
receptors. This is apoptosis pathway activation
directly from the extrinsic pathway, called caspase.
Metabolic disorders of intracellular reactive oxygen
species
or overload can cause damage to t h e
mitochondria, which produce cytochrome c release
and the activation of caspase-9. Caspase-9
reactivates further trigger activation of caspase-3
and apoptosis. High apoptosis hemeostasis systems
cannot keep the organ systems, causing multiple
organ dysfunction syndrome (MODS). Organ system
failure is
very harmful to humans and can even
cause death (Caspian, 2016). Expressions of
Caspase-3 were examined in the liver and spleen of
the rat (Rattus norvegicus) where the apoptosis
occurred.
2 METHODS
The research was done in a purely experimental
laboratory using a true experimental research
design. Post-tests only Control Group Design (data
retrieval is performed after the given treatment) and
compared with the control group.
The research was performed in, the Animal
Models Department of Biochemistry, Microbiology
Clinic Laboratory, Dr. Sutomo Hospital as a place of
clinical isolation for the culture of S. pyogenes and
A. baumannii. The Anatomic Pathology Laboratory
Faculty of Medicine Airlangga University processed
checks and the observation of the expression of
caspase-3 in organ livers and lien on the Rattus
Norvegicus. The research was carried out over three
months. (October 2017–December 2017).
The object of the research is the white R.
norvegicus male wistar strain, aged 3 months with a
weight of 200–250 grams. Four R. norvegicus were
used in this study
Pure liquid bacteria was isolated from S.
pyogenes at
A. baumannii laboratory clinical microbiology of
the Dr. Sutomo Hospital. Expressions of caspase-3 in
rats’ livers and spleen who had infection with
bacteria S.pyogenes and A.baumannii are detected
by IHC method (Immunohistochemistry) using the
caspase-3 p12 subunits of antibodies.
1.
Treatment for animal models:
R. norvegicus were given injections in the
peritoniumnya with bacteria S. pyogenes and A.
baumanni. Each anesthetic had 2.52mg and 0.25mg
of ketamine dexamethasone, followed by an
injection of bacteria S. pyogenes and A. baumannii
of which the doses were 1x109 per CFU/Rat. After
24 hours, if the rat was not dead, it was dissected
and the liver and spleen were taken.
2.
Tissue Preparation:
The liver and spleen were removed from the
treated rats and fixed with 10% formalin. Further
cutting of the organs at 1x1x2cm continued using
paraffin blocks that are used in the pathology
laboratory.
3.
Observation of Caspase-3 expression
Observation on the expression of caspase-3 in
rats liver and spleen was conducted with primary
antibody caspase-3 p12 subunits. Expressions of
caspase-3 were observed using the IHC method by
using caspase-3 p12 subunit primary antibodies. The
cells expressing caspase-3 were counted within five
fields of view x1000 magnification and the numbers
were compared between two bacteria-treated rats.
Cell expression of caspase-3 were calculated on an
examination under microscope light with
magnification 100x objective with five field of view
and magnification of 10x to 40x and photographed
for comparison of each liver and spleen.
3 RESULTS
3.1 The results of the expression of
Caspase-3 in the liver
The number of cells expressing results obtained
demonstrated that the average number of
expressions of caspase-3 in the group injected with
A. baumannii is higher than the group injected with
S. pyogenes and the control group.
Table 1: The Distribution of liver cells expressing caspase-
3 Note SD is Standard Deviation.
Percentage
of cell
C(n=4) S. pyogenes
A
.baumanni
i
X± SD 9.5 ± 2.38 20.75 ± 7.136 48 ± 21.74
Median 9.5 22.5 48
Min - Max 7
–
12 11
–
27 26
–
70
The number of cells expressing caspase-3 in the
livers of rats infected with A. baumannii was higher
than those infected with S. pyogenes
Differences of Caspase-3 Expression in Liver and Spleen of Rattus norvegicus Infected with Streptococcus pyogenes and Acinetobacter
baumannii
259
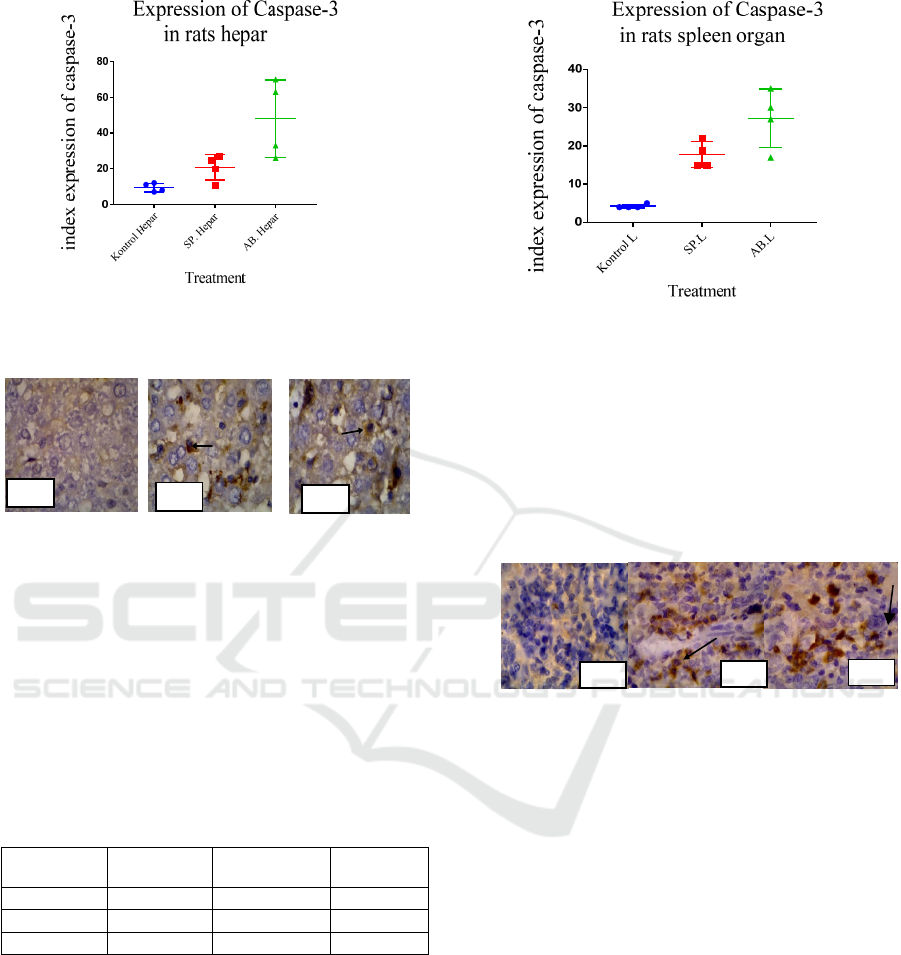
Figure 1: Box plot of the number of cells express caspase-
3 in the liver infected with A. baumannii and S. pyogenes.
Figure 2: No cell expression caspase-3 in the liver control
group, Figure 3: Expression of caspase-3 in rats’ liver
Hepatocyte Cell Group A.baumannii x1000 zoom. Figure
4: Expression of caspase-3 in rats liver Hepatocyte Cell
Group S.pyogenes x1000 zoom.
3.2 The results of the expression of
caspase-3 in the spleen
From the results obtained, the average number of
expressions of caspase-3 in the group infected with
A. baumannii is higher than the group infected with
S. pyogenes and the control group.
Table 2 : Note SD is Standard Deviation
Percentage
of cell
K(n=4) S. pyogenes
A
.baumanni
i
X± SD 4.25 ± 0.5 17.75 ± 7.136 27.25 ± 7.58
Median 4 17 28.5
Min - Max 4
–
5 15
–
22 17
–
35
Based on the data in the table above, it can be noted
that the expression of caspase-3 in rats in the group
infected with A.baumannii is the highest, i.e. SD
27.25 ± 7.58, while the expression of caspase-3 in
the group of rats’ spleens infected with S. pyogenes
is 17.75 ± 3,403.
Figure 5: Box-plot the number of expression of Caspase-3 in
rats’ spleens.
The highest median value, i.e. the A.baumannii
group was 28.5, the second was the S.pyogenes
group at 17, and the control group was 4.
Description of caspase-3 expression was observed
under the light microscope with a magnification of
x100 and x1000 and the x% fields of view are as
follows:
Figure 6: Expression of caspase-3 in the spleen control
group, Figure 7: Expression of caspase-3 on rat
lymphocyte cells spleen Group A.baumannii x1000 zoom,
and Figure 8: Expression of caspase-3 in the spleen of rats
infected with S.pyogenes magnification x1000.
3.3 Expression of Caspase-3 in the liver
and spleens of rats infected with
A.baumannii and S.pyogenes
Based on the averages of cells, those that expressed
caspase-3 in the livers and spleens of rats infected
with A.baumanii and S.pyogenes showed that the
group infected with A.baumannii was higher (48)
than that of rats infected with S.pyogenes (22.5) and
the control group (9.5). The average number of cells
expressing caspase-3 in the spleens of rats infected
with A.baumanii was higher (28.5), compared with
that of rat infected with S.pyogenes (17) and the
control group, which was only 4 as shown in Figure
9.
F2
F3
F4
F6
F7
F8
ICPS 2018 - 2nd International Conference Postgraduate School
260
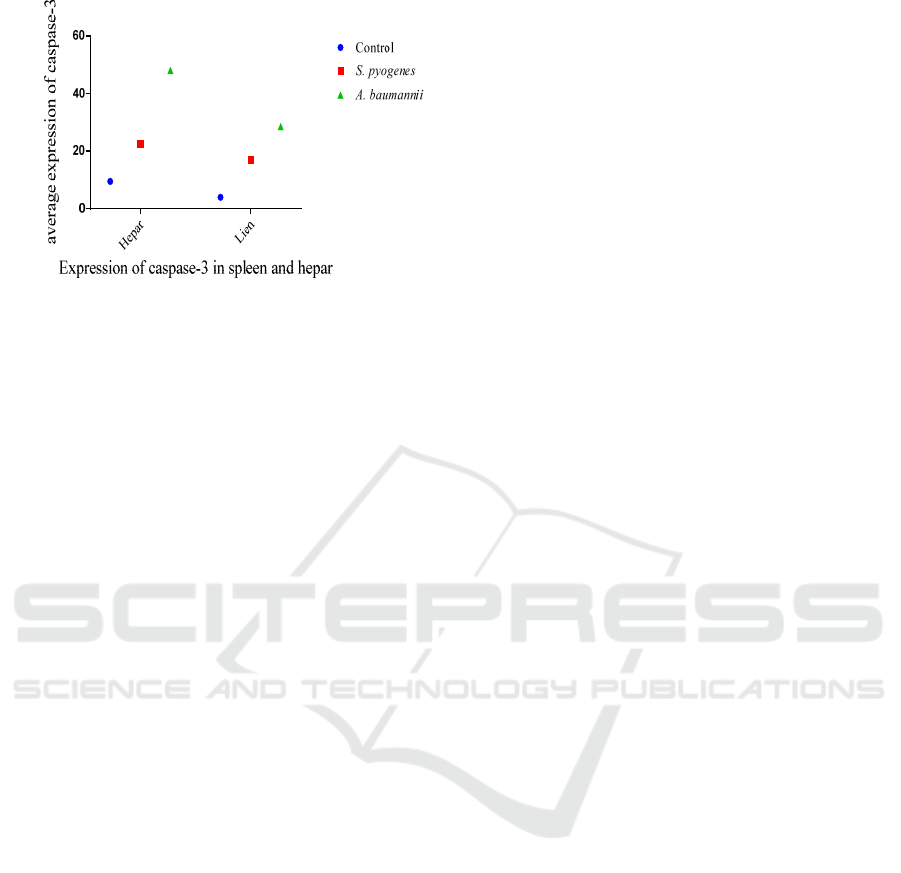
Figure 9: Box-plot Average number expression of Caspase-
3 in rats’ spleens and livers.
4 DISCUSSION
Apoptosis as a type of cell death is highly organized
and genetically controlled. It is characterized by a
number of different morphological changes, such as
cell condensation and marginalization, the shrinking
of cells, and plasma membrane blebbing. This is
accompanied by biochemical features, such as DNA
fragmentation, changes in membrane (e.g. exposure
to fosfatidilerin on the outside of the plasma
membrane), and specific cell protein degradation, as
a result of the activation of a massive amount of
intracellular protease and endonuclease (Guicciardi
and Scratch, 2005). The cleavage of caspase was
mediated by caspase-3 and caspase-7, while the last
two caspase activations are generally a function of
initiator caspase. Initiator caspase’s apoptosis signal
pathway determines, after activation of caspase
executor, that caspase-3 and caspase-7 can process
at least 100 proteins. The cleavage of a caspase-3
substrate can lead to profit or loss of the function of
proteins, ultimately causing cellular changes
associated with apoptosis (Rogers et al., 2015).
1. The death of rats due to A.baumannii and S.
pyogenes.
A. baumannii produces endotoxin as OmpA capsule
(outer membrane protein A) induces apoptosis in
human laryngeal epithelial cells. OmpA is purified
and localized and the mitochondria and apoptosis
are induced through the release of the proapoptotic
cytochrome c molecules and the driving factors of
apoptosis, suggesting that this is the path where the
A.baumannii induces damage to the cells of the
respiratory tract during infection (Peng et al., 2016).
Gram-negative can cause the onset of sepsis and
sepsis shock (Girardt et al., 2016).
Streptolisin O is the basic nature of the toxin beta-
hemolysis with toxins from Gram-positive bacteria
S.pyogenes. Streptolisin O is potentially cell poison
affecting many cell types including neutrophil,
platelets, and organella subsel. This toxin is capable
of producing a large cellular immune response that
can lead to fatal toxic shock (Regnier et al., 2016).
S. pyogenes is a species of gram-positive bacteria
that contain peptidoglycan cell walls and
lipoteichoic acid (LTA) discovered by the immune
system as the PAMPs, is in line for the bacteria
S.pyogenes as a TLR-Peptidoglycan and LTA
interaction with TLR-2 produces a signalling
pathway via the adapter MyD88 and TRIF
activation that can trigger the formation of NF-kB
and cytokines expression of MAPKs so it can be
(Pyrshev et al., 2017).
2.
Expression of caspase-3 in livers of rats
infected with A. baumannii and S. pyogenes
The increased of caspase-3 expression in the livers
of rats infected with A.baumanni and S. pyogenes
indicate the death of cells due to apoptosis but no
damage to the organ. Hepatocyte death is common
in the aftermath of inflammatory disease in the liver.
An increase of apoptosis in the liver can be caused
by a high inflammatory process, which triggers the
apoptotic hepatocyte cell to cause most of the
damage to the hepatocytes, mediated by the reactive
oxygen species (ROS) that initiates inflammatory
reactions and the occurrence of apoptosis in
hepatocytes (Rinaldi, 2014). In hepatocyte damage,
the liver will induce the onset of signals to stimulate
the release of monocyte chemoattractant Chemokin
protein-1 (MCP-1), which will enhance kupffer
cells/macrophages, as well as the release of pro-
inflammatory cytokines, such as interleukin (IL )-1
β, IL-1 and Tumor Necrosis Factor (TNF)-α, which
can enable the Nuclear Factor κB activation (NF-κB
activation) and mitogen-activated protein kinase
(MAPK) (Guicciardi et al., 2013).
3.
Caspase-3 expressions in the spleens of rats
infected with A. baumannii and S. pyogenes.
Potential complications from splenic swelling are
bacterial infections; this is because the spleen
swells, which reduces the number of healthy red
blood cells, platelets, and white blood cells in the
bloodstream, exposing it to the infection (Bronte
and Mikael, 2013).
High bacterial infections activate the immune
system to attack the bacteria present in the blood
(Tan et al., 2017). The spleen will induce
proinflamasi cytokines, such as interleukin (IL)-1 β,
Differences of Caspase-3 Expression in Liver and Spleen of Rattus norvegicus Infected with Streptococcus pyogenes and Acinetobacter
baumannii
261

IL6, the tumor necrosis factor (TNF)-α, γ interferon
(IFN) and the synthesis of nitric oxide (NO).
Patients infected with bacteria undergo
overproduction of proinflammatory cytokines, such
as, TNF-α, IFN-γ, IL-2, ROS and NO. Excessive
TNF-α will increase the production of NO acting as
free radicals. In addition, TNF-α can also increase
the Intercellular Adhesion Molecule-1 (ICAM-1)
and cause obstruction within the brain (Bronte and
Mikael, 2017).
5 CONCLUSION
1. Based on the average of cells expressing
caspase-3 of rats infected with S.pyogenes in
hepar was higher (20.75 ± 7.136) than that in
the spleen (17.75 ± 3.403).
2. Based on the average of cells expressing
caspase-3 of rats infected with A.baumannii in
hepar was higher (48± 21.74) than that in the
spleen (27.25 ±7.58).
3. The highest number of cells expressing
caspase-3 was observed in the group of rats
infected with A. baumannii, compared with
that of the group of rats infected with S.
pyogenes. The liver demonstrated a higher
expression of caspase-3 than the spleen.
REFERENCES
Bigot, S., and Suzana P., 2017, The Influence of two-
partner secretion system on the virulence of
Acinetobacter baumannii Virulence, vol.2, pp 1-2.
Bronte, V., and Mikael J.P., 2013., The Spleen in Local and
Systemic Regulation of Immunity, Immunity Cell
Press, vol.39.
Girardit, T., Thomas R., Fabienne V., and Guillaume M.,
2016., Apoptosis-induced lymphopenia in sepsis and
other severe injuries, Springer Science New York, vol.
22., pp 295-305.
Guicciardi, M.E., and Gores G.J., 2005., Apoptosis : A
Mechanism of acute and chronic liver injury, Rececn
Advances In Basic Science, vol. 54., pp 1024-1033.
Norhamdani, 2004, Acinetobacter Baumannii
Hemaglutinasi activity of bacteria derived from clinical
and Environmental Specimens, Journal of medicine
Brawijaya, vol.20, pp 105-109.
Peng, C.H, Xian W.y, Jing Han, Lin Liu, Cheng Zhang, Jing
X., Jiu R.N., and Xiang Y.Z., 2016, Preliminary study
abaout the mechanism of IL-33 acting on neutrophil
apoptosis in rates with Acinetobacter baumannii
Pneumonia, Intenational Journal Clin Exp Med., vol. 9,
no.6, pp 8934-8944.
Ramachandran. G, 2017, gram-positive & gram negative
bacterial toxins in sepsis virulence vol.5, no.1 pp.213-
218
Regnier, E., Philippe A.G., and Guillaume O., 2016,
Superoxide anions produced by Streptococcus
pyogenes group A-stimulated keratinocytes are
responsible for cellular necrosis and bacterial growth
inhibition, Innate Immunity, Sagepub Journal
Permissions, vol. 22, pp 113-123.
Rinaldi, W.A., 2014. The potential granting of Propolis
Against Lymphoblasts in Diameter and number of
White Male Mice Spleen Pulpa (Mud musculus).
Thesis, Faculty Of Veterinary Medicine University Of
Airlangga In Surabaya.
Tan, Y., Kai Z., Xiang T., Timothy K., Luxia W., Zhenghui
G., Murat A., and Chao Z., 2017, Bacterimes and non-
bacteremic pneumonia caused by Acinetobacter
baumannii in ICUs of South China: A clinical and
Microbiology Study, Nature Scientific Reports.
Ulett, G. C., and Elisabeth E.A., 2006, Regulstion of
Apoptosis by Gram-Positive Bacteria: Mechanistic
Diversity and Consequence or Immunity, Curr
Immunol Rev., vol.2, no.2, pp 119-14
ICPS 2018 - 2nd International Conference Postgraduate School
262
