
Dental Cement Physical and Mechanical Properties by In Vivo
Approach
Prihartini Widiyanti
1,2
, Siswanto
2
and Idha Sa’Ada
1
1
Biomedical Engineering Study Program, Department of Physics, Faculty of Science and Technology, Universitas
Airlangga, Indonesia
2
Physics Study Program, Department of Physics, Faculty of Science and Technology, Universitas Airlangga, Indonesia
Keywords: Dental Cements, Tensile Strength, Compressive Strength, Morphological Feature, In Vivo Approach.
Abstract: The current production technology of dental cement material is growing rapidly compared to 50 years ago.
Cement as dental restoration material must be elastic and have a low conductivity property. There are four
kinds of dental cement commonly used in dentistry, namely zinc phosphate cement, polycarboxylate
cement, glass ionomer cement, and zinc oxide and eugenol cement. There is no study about physical and
mechanical properties based on in-vivo condition. This study aims to know the physical and mechanical
properties of dental cement, by the in-vivo approach and using rabbit as an experimental object. Dental
cements used in this research were zinc phosphate cement (ZPC), polycarboxylate cement (PC), glass
ionomer cement (GIC), and zinc oxide and eugenol cement (ZOEC). The method of this study was the
preparation of tools, materials and experimental animals. We used six male - 5 months in age - rabbits.
Before being treated (fill-teeth material insertion), the rabbits were anesthetized by an anaesthetist from the
Animal Hospital, Faculty of Veterinary Medicine, Universitas Airlangga. Then the rabbit teeth were drilled
and formed to become box cavity. Information which can be obtained from this in-vivo experiment is its
physical and mechanical properties i.e. compressive strength, tensile strength, and microstructure of dental
cement. Based on the physical and mechanical characterization value, the best compressive strength was
101.888 MPa and refers to zinc phosphate cement and the best tensile strength value was glass ionomer
cement with 6.555 MPa. The morphological features, mainly surface structure of dental cement, showed
less well sealed for zinc phosphate cement, strongly bonded with teeth for polycarboxylate cement, there
were lumps of unreacted powder particles for glass ionomer cement and there was a very hard lump formed
for zinc oxide and eugenol cement.
1 INTRODUCTION
The technology of dental cement nowadays is
emerging compared to 50 years ago. This condition
facilitates the dentists to have more choice to restore
the broken teeth or even the loose teeth. One of the
dental cement alternatives is by using polymer.
Some scientists have developed the polymer-based
material to be close to the characteristics and
appearance of the natural teeth (Wagh, 2016;
Manappallil JJ, 2016)
The polymer is a long chain molecule consisting
of several repetitions of units (Combe, 2013). Most
of the polymer was used in the industry or medical
area. One example of polymer used in the medical
area is teeth filling material (dental cement). Cement
as a teeth filler should be elastic (low strength
materials). This cement could be synthesized by
mixing the powder material with some liquid. The
cement composition varies in chemical composition,
characteristics, or the usage. This material also has
low conductivity compared to the metal filling
material.
Four types of dental cement are normally used in
dentistry, zinc-phosphate cement, polycarboxylate
cement, glass ionomer cement, and zinc oxide and
eugenol cement (Noort, 2002).
There was a study that synthesized the zinc
phosphate dental cement zinc oxide and phosphate
acid (Wagh AS, 2016). This study results showed
that the mechanical properties on the zinc phosphate
dental cement increased with the increase of
powder-liquid ratio until their mass was the same.
But, it would decrease if the liquid ratio increased in
the composition ratio. The best composition ratio in
274
Widiyanti, P., Siswanto, . and Sa’Ada, I.
Dental Cement Physical and Mechanical Properties by In Vivo Approach.
DOI: 10.5220/0007541302740280
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 274-280
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

the zinc phosphate dental cement was at 60:40
respective to powder and liquid.
The study of the addition of polystyrene as an
additive substance in the dental cement based on
zinc oxide eugenol has been performed. Polystyrene
is a linear polymer that has thermoplastic property.
This material would be melted at a temperature
around 95°C and become viscous solution at 120-
180°C and become liquid above 250°C, then
degraded above 320-350°C. This study concluded
that the addition of polystyrene to the eugenol, as
much as 10%, could give the best mechanical
properties. Fourier Transform Infrared (FT-IR)
spectrophotometer is a device to make a chemical-
physical identification, especially in the qualitative
analysis on the functional group of organic or an-
organic materials based on the absorbance towards
infrared. FTIR test showed that the mixing of
polystyrene and eugenol is a simple mixture.
From that study, there was a lack in the physical
and mechanical properties testing which was no in-
vivo test using living organism yet. This study
would use the rabbit as a living organism for an in-
vivo test. The teeth of the rabbit would be recessed
as in the class III caries. This type of caries usually
occurs on the anterior teeth and could occur on the
medial or distal surface of the incisor or canine.
From the in-vivo test, the information about the
physical and mechanical properties could be
obtained comprising compressive strength, tensile
strength, and microstructure from several types of
dental cements.
2 MATERIALS AND METHODS
2.1 Materials
The first step of this study was the preparation of the
tools to drill and patch the teeth. They were round
bur, fissure bur and tapered bur for class III cavity
preparation, cement spatula for mixing and taking
the dental cement materials, straight probe for
detecting the cavity, dental tweezers for helping to
take the bur eye, debris and cotton, plastic filling
instrument for inserting the dental cement materials
in the cavity, the glass plate and mixing paper for
mixing the materials, and cotton roll for blocking the
saliva.
The materials for dental patching were zinc
phosphate cement, polycarboxylate cement, glass
ionomer cement, zinc oxide and eugenol cement.
Those four types of dental cements were a package
of powder and liquid that could be obtained in the
market.
The preparation of the animal trial started with
six male rabbits with age of five months. Before the
treatment, the rabbit was anesthetized first to know
their condition. To ease the preparation of the cavity
on the rabbit’s teeth, the rabbits were stunned. The
anesthesia was performed by the vet of Universitas
Airlangga as shown in Table 1.
Table 1: Anesthesia of the animal
F1 F2 F3 F4 F5 F6
Weight (kg) 1.8 2 1.5 1.9 1.6 1.7
Xylazine (ml) 0.5 0.5 0.4 0.5 0.4 0.5
Antrophine
(ml)
0.4 0.4 0.3 0.4 0.3 0.4
Ketamine (ml) 0.9 1 0.8 0.9 0.8 0.9
2.2 Testing Sample Preparation
After the preparation of the tools and materials, the
animal trial sample was prepared. Two incisors of
the rabbit were drilled as shown in Figure 1. The
preparation of class III cavity used round bur, fissure
bur and tapered bur. The preparation was only at the
dentin. After the preparation, the cavity was cleaned
with alcohol-dipped cotton. The caries was detected
by using tweezers.
Figure 1: Class III Cavity based on the position of Caries
After the cavity was dry and clean, the patching
process was performed. The patching area was
isolated by cotton roll to prevent the saliva from
inserting the cavity. The patching materials were
prepared in the glass plate based on the usual
procedure and stirred with cement spatula. Specific
to glass ionomer cement, the patching material was
stirred on the mixing paper with plastic cement
Dental Cement Physical and Mechanical Properties by In Vivo Approach
275

spatula. The patching materials were then inserted in
the cavity by using the plastic filling instrument.
After one or two minutes, the patching was pressed
with amalgam stopper and formed.
On the Rabbit I, the cavity was patched by
using zinc phosphate cement. On the Rabbit II, the
cavity was patched by using polycarboxylate
cement. On the Rabbit III, the cavity was patched by
using glass ionomer cement and on the Rabbit IV,
the cavity was patched by using zinc oxide and
eugenol cement. On the Rabbit V, the right cavity
was patched by using zinc phosphate cement and the
left cavity was patched by using polycarboxylate
cement. On the Rabbit VI, the right cavity was
patched by using glass ionomer cement and the left
cavity was patched by using zinc oxide and eugenol
cement. The total of the overall samples were 12
pieces as shown in Table 2.
Table 2: In-Vivo Test Sample
No. Sample
T
yp
e
Amount
(tail)
Dental Cement
1. A 3 zinc phosphate
cement
2. B 3 polycarboxylate
cement
3. C 3 glass ionomer
cement
4. D 3 zinc oxide and
eugenol cement
The categorization of A, B, C, D was based
on the type of cements applied in the tooth cavity
After the patching process, the sample caring
was performed for 21 days to observe the strength of
the patch after patched to the teeth through in-vivo
test. After that, the teeth were alienated using incisor
teeth alienating pliers and characterized.
2.3 The Sample Characterization
The aim of this characterization was to know the
compressive strength, tensile strength and
microstructure of the dental cement.
2.3.1 Compressive Strength
Compressive strength testing was using Autograph.
The load used in this test was 100 kN. From this test,
the compressive strength given to the sample until it
breaks of fractures was obtained. By using equation
(1), the compressive strength could be determined.
A
F
=
τ
(1)
τ is the compressive strength (Pa),
F
the load on
the sample (N), and
A
is area (m
2
).
2.3.2 Tensile Strength
For knowing the stickiness of the dental cement,
tensile strength testing was used by giving a tensile
load directly to the sample. To ensure the sample
held firmly, the tip of the sample was made bigger
than the middle part of the sample. The tensile
strength measurement was using Autograph. By
using equation (2), the tensile strength was
determined.
A
F
TS =
(2)
TS is the tensile strength (Pa), F is the load
(N), and A is the area (m
2
).
2.3.3 Microstructure
To know the microstructure, a Scanning Electron
Microscope (SEM) was used. The sample was
prepared first by coating with a specific material
(gold) in the stub from metal with a diameter of 9
mm. The sample was then inserted into the specimen
chamber and illuminated with the electron beam (20
kV). The reflected electron was detected by the
scintillator detector amplified by an electrical circuit
that could produce a figure from a Cathode Ray
Tube (CRT). The capturing process was performed
after choosing a specific part of the sample with the
correct magnification so that a good and clear image
was obtained. To print the film of the capturing
result, a vacuum evaporator JEOL JEE-4X was used.
3 RESULT
This study performed an in-vivo test by using a
living organism. The in-vivo test gained the
information of the physical and mechanical
properties, such as compressive strength, tensile
strength, and microstructure from zinc phosphate
cement, polycarboxylate cement, glass ionomer
cement), and zinc oxide and eugenol cement.
ICPS 2018 - 2nd International Conference Postgraduate School
276
3.1 Sample Testing Result
This study was performed experimentally by
measuring the mechanical properties, which were
compressive strength and tensile strength. The
sample characterization result is shown in Table 3.
Table 3: The Result of Sample Characterization
No. Sample
Type
Compressive
Strength (MPa)
Tensile
Strength
(MPa)
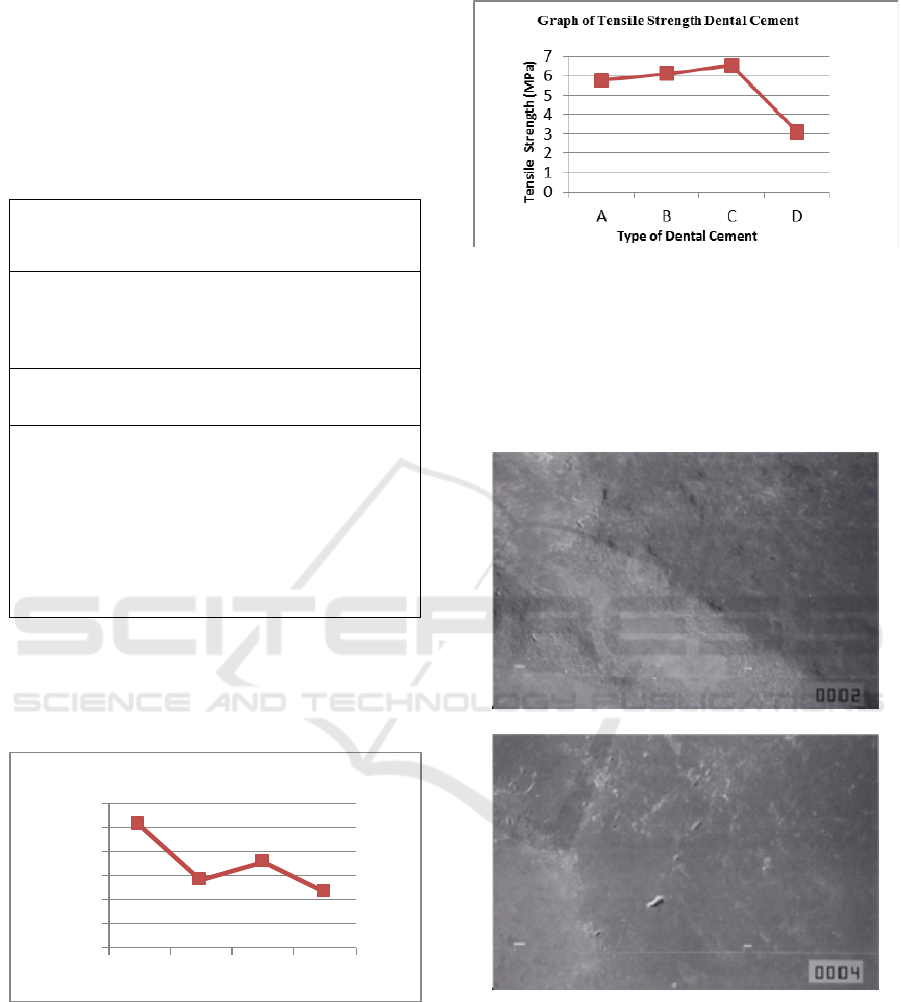
1. A 101.888 5.777
2. B 56.555 6.111
3. C 70.777 6.555
4. D 46.111 3.111
Description :
A = zinc phosphate cement
B = polycarboxylate cement
C = glass ionomer cement
D = zinc oxide and eugenol cement
From the result of sample characterization, there
was a relation between the type of dental cement and
their mechanical properties. The graphs of
compressive strength and tensile strength are shown
in Figures 2 and 3.
0
20
40
60
80
100
120
ABCD
C o m press ive St reng th
(MPa)
Type of Dental Cement
Graph of Compressive Strength Dental
Cement
Figure 2: The Compressive Strength of Several Dental
Cements
Figure 3: The Tensile Strength of Several
Dental Cements
The microstructure of the dental cement could
be observed from the result of SEM. The
microstructure of the dental cement is shown in
Figure 4.
A
B
Dental Cement Physical and Mechanical Properties by In Vivo Approach
277

Figure 4: (a) The microstructure of zinc phosphate cement.
(b) The microstructure of polycarboxylate cement. (c) The
microstructure of glass ionomer cement. (d) The
microstructure of zinc oxide and eugenol cement
4 DISCUSSION
The results on the mechanical properties of the
dental cement show that the best compressive
strength was the zinc phosphate cement at 101.888
MPa and the best tensile strength was glass ionomer
at 6.555 MPa. If manipulated correctly, the zinc
phosphate cement could have the compressive
strength of 104 MPa and tensile strength of 5.5 MPa
(Anusavice, 2003).
The compressive strength and tensile strength
were various based on the ratio of the powder and
liquid. The increase in the strength was obtained by
adding more powder than was recommended and it
was obvious compared to the decrease of the
strength caused by the decrease of the powder in the
mixture. The decrease of the ratio of powder and
liquid would produce a weak cement. The lack or
increase of water content from the liquid would
decrease the tensile strength and compressive
strength of the cement. Like zinc phosphate cement,
glass ionomer cement would be easy to break when
hardening. After that, the recess of the cement could
be thrown away by gouging or breaking the cement
away from the restoration side. This cement is
sensitive to water contamination in the hardening
process. Thus, the restoration side should be coated
to protect the cement from early contact with a
liquid.
The compressive strength from polycarboxylate
cement was lower than the zinc phosphate cement
but had a slightly higher tensile strength. This
cement was not as brittle as zinc phosphate cement,
so that it was more difficult to eliminate the recess
of the cement after the cement hardened.
The mechanical properties of the zinc oxide and
eugenol cement were lower than the other cements.
This cement was hard to manipulate in the mouth
cavity. The thickness of its layer tended to be higher
and the recess of the hardened cement was hard to
remove.
The difference in compressive strength and
tensile strength was caused by the speed of mixing
between the powder and the liquid, the mixture plate
and the temperature of the mixing tools. The speed
of the mixing between powder and liquid could
affect the hardness of the dental cement because the
powder that was mixed with the liquid gradually
with a small amount would increase the working
time and hardness so that it could decrease the heat
produced and allow more powder to be added to the
mixture.
The mixture plate and the temperature of the
mixing tools could also affect the mechanical
properties of the dental cement. The high
temperature on the mixing tools could increase the
hardening reaction from the dental cement. On the
other hand, if the temperature of the mixing tools
was low, then the hardening reaction of the dental
cement could be longer so that the matrix formation
could be slowed down. Besides that, what needs to
be taken into account is the technique of mixing the
powder and liquid. The inappropriate mixing could
cause a crack in the dental cement so that it would
complicate the mechanical properties measurement.
The microstructure of the zinc phosphate cement
in Figure 4(a) showed that the dental cement and the
teeth were not stuck together. When the powder was
mixed with the liquid, the phosphoric acid had
contact with the particle surface and released zinc
ions to the liquid. The aluminium, which has been
bonded to the phosphoric acid, reacts to the zinc and
produces zinc gel as aluminophosphate on the
surface of the residual particles. This hardened
cement is the main structure and consisted of
unreacted zinc oxide particles, coated with a solid
matrix that has not been formed from the zinc
aluminophosphate. Because water affects the acid-
C
D
ICPS 2018 - 2nd International Conference Postgraduate School
278

base reaction, the composition of the liquid should
be maintained to ensure a consistent reaction. The
change in composition and reaction speed could
occur due to the evaporation of the water. So, the
change in composition could affect the reaction.
The microstructure of the polycarboxylate
cement in Figure 4(b) showed that the cement was
bonded tightly to the tooth structure. The hardening
reaction of this cement involved the dissolution of
the particle surface by the acid that released the zinc,
magnesium, and tin ions and merged to the polymer
chain though carboxyl group. These ions react to the
carboxyl group and polyacid chain near them and
form a salt with crosslink while the cement was
hardening. The hardenend cement consisted of non-
uniform matrix gel with a spread of unreacted
particles inside. The microstructure image was
similar to the zinc phosphate cement.
The microstructure of the glass ionomer cement
in Figure 4(c) showed that there was a lump of
powder particles that did not react. When the powder
and the liquid were mixed to form a paste, the glass
particle surface would be dissolved in the acid. The
calcium, aluminium, sodium, and fluorine ions were
released to the watery media. The polyacrylic acid
chain would crosslink with the calcium ions and
form a solid mass. For the next 24 hours, a new
phase was formed in which aluminium ions bond in
the cement mixture and form a brittle cement.
Sodium and fluorine ions did not have a part in the
crosslinking of the cement. Some of the sodium ions
could replace hydrogen ions from the carboxylic
group, and the rest would join the fluorine to form
natrium fluoride that spread evenly in the hardened
cement. Along the hardening process, the
crosslinking phase was also hydrated by the same
water as the medium. The parts that did not react
with the glass particles would be coated by the silica
gels that have been formed during the cation release
from the particles surface. Thus, the hardened
cement consisted of lumps of powder particles that
have not reacted and been surrounded by the silica
gels in the amorphous matrix of calcium hydrate and
a mixture of aluminium salt.
The microstructure of the zinc oxide and eugenol
cement in Figure 4(d) showed that there was a hard
lump. In the right condition, the reaction between
zinc oxide and eugenol resulted in a hard relative
mass. The hardening mechanism of the zinc oxide
eugenol materials consisted of hydrolysis of zinc
oxide and eugenol to form lumps. Zinc acetic
dihydrate accelerated it, that was more soluble than
zinc hydroxide and could give zinc ions faster. The
high temperature could increase the hardening
speed.
The main property of the dental cement is that it
should last in the solubility and disintegration in the
mouth cavity. The cement had continuous contact
with several types of acid produced by the
microorganism and food processing. Some of them
were carried to the mouth as food and beverages. pH
and temperature in the mouth cavity were always
changing. So, no cement could fulfill all desired
ideal characteristics. A cement system is maybe
suitable for one use compared to the other system.
Every condition must be evaluated based on the
environment and biological and mechanical factors.
5 CONCLUSIONS
Based on the in-vivo test, the physical and
mechanical properties were obtained from four types
of dental cements. The mechanical properties were
determined through compressive strength and tensile
strength. The best compressive strength was shown
by zinc phosphate dental cement at 101.888 MPa
and tensile strength from glass ionomer cement at
6.555 MPa. The dental cement from zinc oxide and
eugenol had the lowest physical properties compared
to the other dental cements.
ACKNOWLEDGEMENTS
I would like to acknowledge with appreciation to the
Faculty of Veterinary and Animal Hospital Universitas
Airlangga for the facilitation and support on the in vivo
study of this research.
REFERENCES
Anusavice J.K., Shen C, Rawis HR, 2013. Phillips of
Science of Dental Materials, 12
ed
, Saunders Elsevier,
Missouri, 3-16; 48- 68; 307- 339.
Combe E, Trevor Burke FJ, Douglas W. Bernard, 2013.
Dental Biomaterials, Springer US, 222-276
Chung DDL, 2004. Use of polymers for cement-based
structural materials, Journal of Material Science 39,
2973 – 2978
Manappallil JJ,2016. Basic Dental Material, Jaypee
Brothers Medical Publishers, Ltd, Nepal, 3-61; 84-
167.
Noort, R.V.,2002, Introduction to Dental Material, 2nd
ed, Mosby, London, 6-12
Nugroho, Dr. Pramono, 2007, Pembuatan Semen Tambal
Gigi dengan Bahan Dasar Polimer, LIPI, Bandung
Dental Cement Physical and Mechanical Properties by In Vivo Approach
279

Wagh AS, 2016. Chemically Bonded Phosphate ceramics,
Twenty – First Century Materials with Diverse
Applications, 2
nd
edition, Elsevier, Cambridge, 133-
138
ICPS 2018 - 2nd International Conference Postgraduate School
280
