
Correlation between Parasite Density with TNF-α and IL-10 in
Plasmodium Falciparum Infected Patients in East Sumba District,
East Nusa Tenggara Province
Akhmad Mubarok
1
, Heny Arwati
2
and Yoes Prijatna Dachlan
2
1
Department of ImmunologyPostgraduate School, Universitas Airlangga, Airlangga Street 4-6, Surabaya, Indonesia
2
Department of Parasitology Faculty of Medicine, UniversitasAirlangga, Surabaya, Indonesia
Keywords: Parasite density, Plasmodium falciparum, TNF-α, IL-10.
Abstract: Introduction: Malaria is caused by female mosquito Anopheles bite transmitting malaria parasite sporozoite
stage into the human body. According to Word Health Organization (WHO) due to malaria was 429.000
shows case causing malaria death, this need commitment of every country to overcome malaria. Indonesia is
still a malaria endemic area and includes a country with a high risk of malaria. Population migration from
malaria endemic areas to non-endemic areas of malaria is responsible for malaria transmission, especially in
Papua, West Papua, Maluku, North Maluku and East Nusa Tenggara. Malaria cases in East Nusa Tenggara
Province 7,05% per 1.000 opulation based on annual parasite incidence (API) and 36,039 positive. East
Sumba 12,84% per 1.000 population. Methods: A descriptive cross sectional study was conducted in East
Sumba district using the Logical Framework approach. Twenty two people were involved in this study with
ranging age 4 to 50 years old. Parasite density was examined by counting parasites on Giemsa-stained thick
smears under light microscope. Plasma level of TNF-α and Interleukin-10 were measured by using enzyme
linked immunosorbent assay (ELISA) Result: Significant values considered at p<0.05 The result show that
parasite density7.72 ±9.51/µl.TNF-α27.14 ± 50.22pg/ml IL-10 2.20± 3.23pg/ml Conclusion: The result
from this study conclude parasitedensity increases of TNF-αand IL-10 cytokine.
1 INTRODUCTION
Malaria one of deadly tropical parasite disease
caused by the genusPlasmodium, transmitted by the
femaleAnopheles bites they transmitted parasite into
the human (Mota et al, 2017).Data in 2015 people
death case cause malaria 429.000, need commitment
every country to against malaria (WHO,
2016).Indonesia one ofcountry with a high risk of
malaria endemic areaspecially in Papua, West
Papua, Maluku, North Maluku and East Nusa
Tenggara.East Sumba district part of East Nusa
Tenggara with high risk of malaria (Kemenkes,
2016).
Glycosylphosphatidylinositol (GPI) is malria
toxin that released along with merozoit and
hemozoin when P. falciparum¬ schizont-infected
erythrocytes rupture causing severe malaria
pathology through stimulation of pro-inflamatory
responses from NK cells and macrophages as
immune cells during innate immunity. Immune
response induced by GPI is mediated by pattern
recognition receptors such as TLR2 and TLR42
(Dunst et al, 2017). Intraerythrocytic malaria
parasite antigens trigger the early immune response
and cytokines production such as TNF-α, IL-1 and
IFN- from macrophages (Mana et al, 19910). The
interleukin-10 (IL-10) is an anti-inflamatory
cytokine produced by monocyte/lymphocyte which
has been shown to inhibit TNF-α (Burdin et al,
1994) protects against severe malaria anemia (SMA)
and cerebral malaria (Terazzaset al 2017).
IL-10 cytokines are found in plasma, produced
by monocytes, Th2 cells cyteand B cells, inhibiting
cytokine production in Th1 and CD8 + cells.IL-10
anti-inflammatory cytokine protects against severe
malaria anemia (SMA) withcerebral malaria
(Terazzaset al 2017).
During inflammation production of TNF-α is
increased. The dual of cytokines especially TNF-α
and IL-10 in the right level play role in protection
and healing (Irawati et al, 2008).
412
Mubarok, A., Arwati, H. and Dahlan, Y.
Correlation between Parasite Density with TNF-Î
´
s and IL-10 in Plasmodium Falciparum Infected Patients in East Sumba District, East Nusa Tenggara Province.
DOI: 10.5220/0007544004120417
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 412-417
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

In this study, parasite density, plasma level of
TNF-α and IL-10 of P. falciparum-infected patient
and residents in East Sumba District of East Nusa
Tenggara Province were measured to find out the
correlation between parasite density and both
cytokines to determine the immune status of the
patients.
2 MATERIAL AND METHODS
2.1 Location and Samples Collection
This research was done in East Sumba district of the
East Nusa Tenggara Province. This district that
located in tropical region has rainy season during
January-April and the rest was dry season, causing
this region classified as dry area (BPS Sumba Timur,
2016).
Blood were collected from P.falciparum-infected
patients who seeked medication in Lindimara
Hospital and Public Health Service (Puskesmas),
and from malaria-suspected residents who were
developing fever during samples collection. Only
P.falciparum-infected blood were used in this study.
Three milli liters (mL) of blood were collected from
median cubital vein and transffered to the heparin-
containing tube, furthermore, plasma were used to
measured the cytokines levels.
2.2 Microscopy Diagnosis and
Determination of Parasite Density
Microcopy examination was done on Giemsa-
stained thick and thin blood film using light
microscop under 1000x magnification with oil
immersion to detect and identify the species of
P.falciparum. Parasite density were counted per 500
leukocyte based on the following formula:
=
∑
(1)
Blood drops on the surface glass object, flattened
using the other glass object, thin blood smear attach
the glass objectwith a 45 degree in the blood, push
blood untill tip on ovale, thick and thin blood
fixation with metil alcohol dried and then staining
used giemsa.
2.3 Procedure of Giemsa Staining
The dried blood preparation fixation with methanol,
glass objects are placed on the stain rack, prepared
giemsa solution by mixing 3 cc giemsastock and 97
cc buffer solution, poured 3% giemsa solution to
cover the surface of the glass object, leave on for 30
– 45minute, poured clean water slowly on the glass
object until clean, dried and than staining used
giemsa.
2.4 Enzyme-Linked Immunosorbent
Assay (ELISA)
Plasma levels of TNF-α and IL-10 were measured
by using ELISA according to the manufacturer’s
protocol (Elabscience, USA), with all samples
running in a single assay. The ELISA was performed
and analysed by a single operator, and standard
curves were derived from cytokine standards.
Optical density (OD) value were measured at 450
nm immediately.
Add 100 µL sample of standard or plasma to
each well. Incubate for 90 minutes, remove the
liquid. Add 100µL of biotinylated detection antibodi
(Ab). Incubate for one hour at 37 degree, aspirate
and wash for three times, add 100 µL of HRP
conjugate. Incubate for 30 minutes at 37 degree,
aspirate and wash for 5 times, add 90 µL of substrate
reagent. Incubate for 15 minutes at 37 degree, add
50 µL of stop solution. Determine the optical density
(OD) value at 450 nm immdiately.
2.5 Statistical Analysis
All the statistical analyses were done using statistical
package for social science (SPSS). The normality
data was determine by Kolmogorov Smirnov test
with p > 0,05. Further analysis was using Pearson
corellation test to determine the correlation between
parasite density, TNF-α, IL-10 and the ratio of both
cytokines.
3 COPYRIGHT FORM
3.1 Microscopy Diagnosis of P.
falciparum Infection
Microcopy examination was done on Giemsa-
stained thick and thin blood film using light
microscop under 1000x magnification with oil
immersion to detect and identify the species of
P.falciparum. Parasite density were counted per 500
leucocyte based on the following formula:
=
∑
(2)
Correlation between Parasite Density with TNF-Î
´
s and IL-10 in Plasmodium Falciparum Infected Patients in East Sumba District, East Nusa
Tenggara Province
413
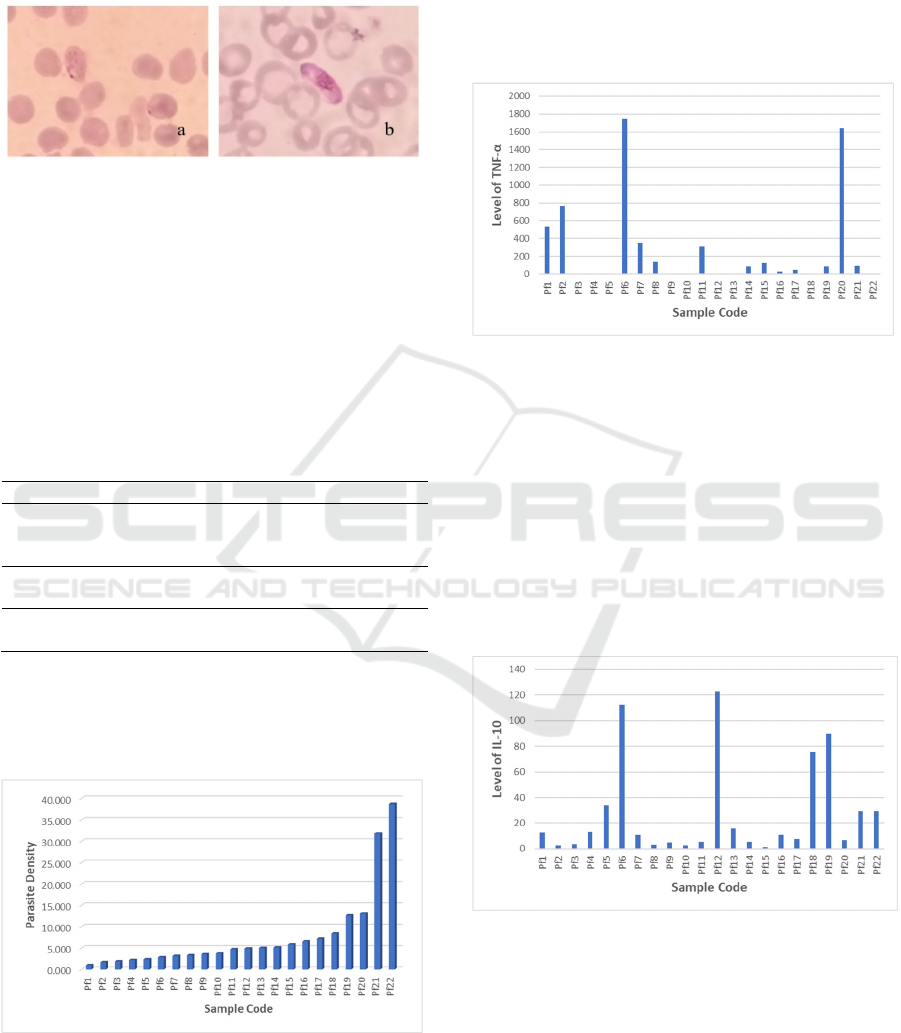
Mycroscopy diagnosis of Giemsa-stained thin
films resulting in 22 out of 46 samples were
positive infected with P. falciparum,19 P. vivax and
5 mix of both species. Only P. falciparum positive
samples were used in this study.
Figure 1: Ringform (a) and gametocyte (b) stages of P.
falciparum on Giemsa-thin blood film
3.2 Characteristics of Subjects
The results of the characteristics subjects are
presented in Table 1. Mean age of malaria positive
group P.falciparum 16,64 years and negative group
24,10 years. Percentage of gender malaria positive
group 50% male, 50% female and malaria negative
group 30% male, 70% female.
Table 1: Characteristics of subjects
Total (%) Total (%)
Number of
the subject
Positive group
P.falciparum
(n = 22)
Negative group
P.falciparum
(n = 10)
Age (Year),
Mean ±SD
16,64 ±10,67 24,10 ± 5,74
Male 11(50,0) 3(30,0)
Female 11
(
50,0
)
7
(
70,0
)
3.3 Parasite Density
The calculation of parasite density P.falciparum are
presented in Figure 2 below.
Figure 2: Parasite density in subject infected with
P.falciparum
The highest parasite density was 38,768
parasites/μL while the lowest was 1,008
parasites/μL.
3.4 Plasma level of TNF-α
The results of measurements of TNF-α levels are
shown in Figure 3 below.
Figure 3: Plasma level of TNF-α in P. falciparum-infected
subjects
The highest levels of TNF-α were found in Pf6
1750.94 pg / mL and Pf20 1641.59 pg / mL samples.
The lowest TNF-α levels Pf3, Pf4, Pf5, Pf9, Pf10,
Pf12, Pf13, Pf18, Pf22 were below the standard
absorbance values of the ELISA measurement.
3.5 Plasma Level of IL-10
The plasma level of IL-10 by ELISA is shown in
Figure 4.
Figure 4: Plasma level of IL-10 levels in P. falciparum-
infected subjects
The highest levels of IL-10 were found in
samples of Pf6 with 112.04 pg / mL and Pf12 with
122.76 pg / mL. The lowest level of IL-10 were Pf2
with 2.52 pg / mL, Pf10 with 2.40 pg / mL and Pf15
with 1.05 pg / mL.
ICPS 2018 - 2nd International Conference Postgraduate School
414
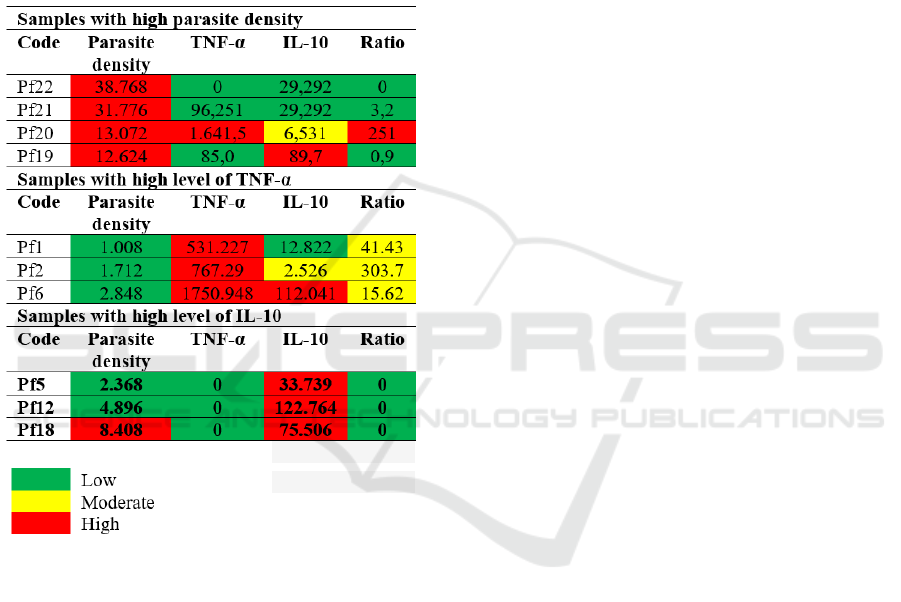
The levels of parasite density, TNF-α and IL-10
vary widely, therefore, to explain the correlation
between parasite density and the levels of TNF-α
and IL-10, the discussion is grouped into 3 groups
and picked up several samples as follows:
1. Samples with high parasite density. 2. Samples
with high TNF-α level or high ratio of TNF-α: IL-
10, and 3. Samples with high IL-10 levels or low
TNF-α: IL-10 ratio.
Table 2: Grouping of representative samples with high
parasite densities, high TNF-α and high IL-10 levels
4 DISCUSSION
The higher levels of parasite density were observed
in uncomplicated P.falciparum infection compared
to the severe malaria infection. In the present study
high parasite density was followed by high TNF-α
and IL-10 levels. Activation of Th1 and Th2 balance
for parasite killing (Irawati, 2008). Several studies
have shown the involvement of pro-inflammatory
cytokine in patogenesis of severe falciparum malaria
where high plasma TNF-α and IL-10 show cerebral
malaria (CM) and severe malaria anemia (SMA)
(Parera, et al 2013)
Several studies indicated TNF-α is a critical
mediators of malarial fever P.falciparum. TNF-α
released in intermittent burst with the schizont
rupture. Not all proinflammatory cytokines are
equally relevant for the development of cerebral
malaria (Angulo, 2002). Cytokines play an
important role in human immune responses to
malarial disease. Balance between pro and anti
inflamatory cytokine are differ in each stage of
Plasmodium infection (Andrade, 2010).
4.1 Samples with High Parasite Density
There are 4 samples categorized as having high
parasite densities (> 10,000/µl blood) with different
levels of TNF-α and IL-10 as listed in the Table 2.
Subject Pf21
This subject with high parasite density has
low TNF-α and IL-10 levels, make the ratio of
TNF-α : IL-10 also low (0). The low anti-
inflammatory response managed the host
immunity to suppress the levels of
proinflammatory cytokine (TNF-α) to the level
below standard (0). Possibility in defecting or
mutations in TNF-α encoding gene has occured
that can result in polymorphisms of the gene
(Qidwai and Khan, 2011).
Subject Pf20
This subject has high parasite density with
high TNF-α levels but moderate IL-10 level, thus
the ratio of TNF-α : IL-10 is high. The blood
sample was collected at the Baing Health Center
when the subject was seeking medication due to
suffering from fever. Microscopy diagnosis has
confirmed the positive infection of P. falciparum.
The immune response of patient is indicated by
high levels of TNF-α. Fever is a clinical
manifestation of the TNF-α response. Fever and
TNF-α maintain parasitic density within safe
limits. Such immunity explains a host defense
mechanism that depends on parasite density
(Kwiatkowski 1989; 1991; 1994). TNF-α has
several other biological effects, such as the
deployment of neutrophils and monocytes to the
site of infection and activating these cells to
exclude pathogens, stimulating the expression of
vascular endothelial cell adhesion molecules for
leukocytes, stimulating macrophages to secrete
chemokines and inducing chemotactic and
leukocyte deployment, stimulating the
hypothalamus inducing fever (Bratawidjaja,
2014). High parasitic density induces TNF-α
production very strongly. The excessive level of
Correlation between Parasite Density with TNF-Î
´
s and IL-10 in Plasmodium Falciparum Infected Patients in East Sumba District, East Nusa
Tenggara Province
415

TNF-α indicate that parasites escape the immune
mechanism. The host fights infection by
producing anti-inflammatory cytokines, IL-10,
but is unable to kill parasites mimicking the
immunotolerance against pathogen. These
conditions can lead to splenomegaly and
hepatomegaly and maybe specific in East
Sumba Regency.
Subject Pf19
The host responds to infection by producing
high level of IL-10 to suppress TNF-α
production, however, IL-10 produced by Th2
cells is unable to help B cells to produce anti-
parasitic antibodies that are strong enough to
eliminate parasites, so parasites continue to
increase. A research conducted by Shabani et al
(2017) described that density of P. falciparum
which >10,000 parasites µl of blood can cause
Cerebral Malaria (CM) and Severe Malaria
Anemia (SMA). In anemia, the parasite density
increases (Maina et al, 2010). P. falciparum
infects old erythrocytes. When erythrocytes
infected with schizont stage rupture release
merozoites to invade other erythrocytes, thus the
more erythrocytes infected by the schizont result
in reducing the number of erythrocytes. On the
other hand, the production of new erythrocytes is
not as fast as the invation of parasites. Parasitic
density is related to age, and clinical malaria
such as fever, chills, headache and splenomegaly
are associated with parasite density (Pryblyski et
al, 1999). Peripheral parasitic density is also
associated with plasma TNF-α level in pregnant
women (Ifeanyichukwu et al, 2017).
4.2 Samples with High TNF-α Level
Subject Pf code 1
This subject has low parasite density, high
TNF-α levels and moderate IL-10 levels, thus the
ratio of TNF-α:IL-10 is moderate. High TNF-α
level plays a role in parasite killing mechanism,
because TNF-α levels can inhibit parasite growth
(Kwiatkowski, 1991), causing low parasitic
density. Moderate levels of IL-10 indicate the
host responds against infection to balance the
TNF-α. Subject Pf2 has similar immune status to
Pf 1.
Subject Pf6
This subject has low parasite density, but
high level of TNF-α and IL-10 and moderate
ratio of both cytokines. This situation shows that
the host is fighting infection by producing IL-10
to compensate for TNF-α production. Parasite
killing mechanism has also occurred. The
presence of an anti-inflammatory response
indicates a tendency for patients to recover from
malaria infection.
4.3 Sample Group with High IL-10
Level
Subjects Pf5, Pf12 and Pf18 had defective TNF-α
coding genes (Qidwai and Khan, 2011), causing
very low TNF-α levels and unreadable by the system
on measurements with the ELISA method. High
respond of IL-10 indicates a strong fight against
infection followed by a decrease in parasite density,
thus indicates healing is very likely.
5 CONCLUSIONS
In summary, proinflammatory cytokine and anti-
inflammatory cytokine are both required for
adequate protection, Th-1 cytokine are important in
controlling early parasite malaria, although they
need to be counterbalanced later in the infection by a
Th-2 response which leads to antibody production.
REFERENCES
Angulo, I and Fresno, M. 2002. Cytokine in The
Pathogenesis of and Protection against Malaria.
Clinical and Diagnostic Laboratory Immunlogy.
American Society for Microbiology. Vol.9, No.6
Badan Pusat Statistik. 2017. Sumba Timur Dalam Angka.
Dachlan, Yoes Prijatna. 2013. Imunologi Malaria :Sistem
Imun Innate dan Adaptive pada Malaria. Rumah Sakit
Penyakit Tropik Infeksi. Univerersitas Airlangga.
Surabaya.
Harijanto, P.N. Nugroho, Agung. Gunawan, Carta A.
2012. Malaria dari Molekul ke Klinis. Edisi 2.
Penerbit Buku Kedokteran EGC. Jakarta.
Irawati, L. Acang, Nusirwan. Irawati, Nuzulia. 2008.
Ekspresi Tumor Necrosis Factor -Alfa (TNF-α) dan
Interleukin 10 (IL-10) pada Infeksi Falciprum.
Majalah Kedokteran Andalas No.1 Vol.32
Mota, M. Rodriguez, Ana. 2017. Malaria Immune
Response to Infection and Vaccination. Springer
International Publishing. Switzerland.
White, Nicholas J. Sasithon Pukritakamee, Tran Tinh
Hien, M Abul Faiz, Olugbenga A Mokuolu, Anjen M
Dondorp. 2014. Lancet Journal. Malaria. Vol.383
February 22.
ICPS 2018 - 2nd International Conference Postgraduate School
416

Terrazas, Cesar, James C Stock, Jennifer Kimble, Ellen
Morreti, Sanjay Varikuti and Abhay R. Satoskar.
2017. The Role of MIF in Parasitic Infections. USA.
World Health Organization (WHO). 2016. World Malaria
Report. Geneva. Switzerland
Parera M.K, Herath N.P, Pathirana L, Phone Kyaw M,
Alles H.K, Mendis K.N, Premawansa S,
HandunnettiS.M. 2013. Association of high plasma
TNF-alpha levels and TNF-alpha/IL-10 ratios with
TNF2 allele in severe P.falciparum malaria patients in
Sri Lanka. Phatogen and global health. Vol.107. No.1
Correlation between Parasite Density with TNF-Î
´
s and IL-10 in Plasmodium Falciparum Infected Patients in East Sumba District, East Nusa
Tenggara Province
417
