
The Effect of Seawater on the Quantity of Dental Pulped DNA in
Forensic Odontology Identification
Nazaratun Thaiyibah
1
, Amalia Rozaiza Ightikhoma
1
and Ahmad Yudianto
1,2
1
Forensic Science Program, Postgraduate School, Universitas Airlangga Campus B, 4-6 Airlangga Rd, 60286
Surabaya,Indonesia
2
Human Genetic Laboratory, Institute of Tropical Disease, Universitas Airlangga Surabaya, Indonesia
Keywords: Dental Pulp DNA, Forensic odontology, Seawater
Abstract: The identification of forensic odontology on victims of sea natural disasters is highly efficient. Its contrast
with the identification of soft tissue that are susceptible to damage is a consequence of seawater exposure.
Seawater affects the decomposition of corpses and creates potential difference due to salinity (content of salt)
in the water. The identification of odontology can consist of DNA analysis of the dental pulp, because the
dental pulp is shielded by hard tissue such as dentine and enamel which are makes the pulp capable to protect
the DNA, but the enamel is semipermeable (water permeable), which can affect the extraction of DNA degree
and its purity. This study aims to determine the effectiveness of seawater to the quantity of teeth pulp DNA.
The research was conducted experimentally by observing and explaining situations that occurred (cause and
effect) in dental pulp DNA quantity exposure to seawater from 1 day and 7 days using spectrophotometer
method with wavelengths of 260 nm and 280 nm. The results of this study indicated that the waters of the
Lombok Strait with 28.74 ‰ of salinity degraded the DNA content of the dental pulp, but the DNA purity
remained stable.
1 INTRODUCTION
Identification of forensic odontology is part of branch
of dentistry that uses dental knowledge for social or
criminal problems for victim identification. Field of
dentistry involves collection and interpretation of
dental evidence and other evidence that related with
criminal. Identification of forensic odontology is
carried out in mass disasters that are naturally
occurring, as well as those caused by human
negligence such as fire, explosion, body decay, or
accidents at sea (Krishan, 2015).
For the victims of mass disasters at sea,
nidification of forensic odontology is very efficient,
while identification of soft tissue easily leads to
broken tissues because of the seawater exposure that
makes decomposition occur more quickly. The
effectiveness of seawater can be seen from the total
of salt or salinity of the water. Research done by Putri
(2016) stated that there is an impact of seawater to
DNA quantity from victims in terms of psoas muscle.
This impacts the identification action because visual
action and fingerprint cannot be used anymore
(Irnamanda, 2016). If accidents at sea occur where
body parts are destroyed, the only specimen needed
for sample is tooth (Datta, 2012).
Tooth is one of human body structure which is
most sturdy and most resistant to bad conditions such
as decomposition, microbes’ action, incineration, and
also environment attack. Therefore, tooth is used as
an identification tool for forensic odontology
investigation. There are pieces information that we
can get from human tooth identification, such as age,
sex, race, facial shape, blood type, and it is also
valuable source of DNA (Rai, 2012). Most forensic
odontology investigation cases might fail because of
insufficient appropriate antemortem records. If no
sufficient amount of antemortem records is not
available, appropriate identification become complex
and the investigators could only obtain the biological
profile of molecular system, which is the DNA that
expose the true identity of someone (Datta, 2012).
Deoxyribonucleic Acid (DNA) genome obtained
from tooth sample can contain about 6 μg to 50 μg of
DNA. Datta (2012) said that Polymer chain reaction
(PCR) allows individual differenciation from others
with high reliability concentration and only 1ng (one
per one billion grams) from DNA target. Deoxyribo
440
Thaiyibah, N., Ightikhoma, A. and Yudianto, A.
The Effect of Seawater on the Quantity of Dental Pulped DNA in Forensic Odontology Identification.
DOI: 10.5220/0007544604400445
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 440-445
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
Nucleic Acid (DNA) abudance quantity can be
extracted from tooth. This is the important advantage
from DNA analysis, which is that the tooth DNA is
contained on the tooth pulp layer.
Tooth pulp mitochondria DNA is obtained from
tooth pulp, which is a connective tissue that is rich in
blood vessels and nerves contained within the core
layer of the tooth (Girish, 2010). Tooth pulp gets
protection from dentin coating and enamel. This
protection causes tooth pulp DNA to be 100% usable
for gender analysis using polymerase chain reaction
(PCR) analysis after the tooth is heated at temperature
100° C for 15 minutes (Febri, 2013). However, the
research done by Irnamanda (2016) said that there is
seawater influence on accuracy of ABO blood type
determination from tooth pulp. Therefore, the
research of seawater effectiveness on quantity of
DNA pulp tooth from concentrations and purity after
DNA isolation using DNAzol method should be
conducted.
2 MATERIALS AND METHODS
2.1 Materials
This study was carried out in the Laboratory of
Analytical Chemistry of Science Faculty and
Technology. And the DNA was analyzed in Institute
Tropical Disease, Universitas Airlangga Campus C,
Surabaya.
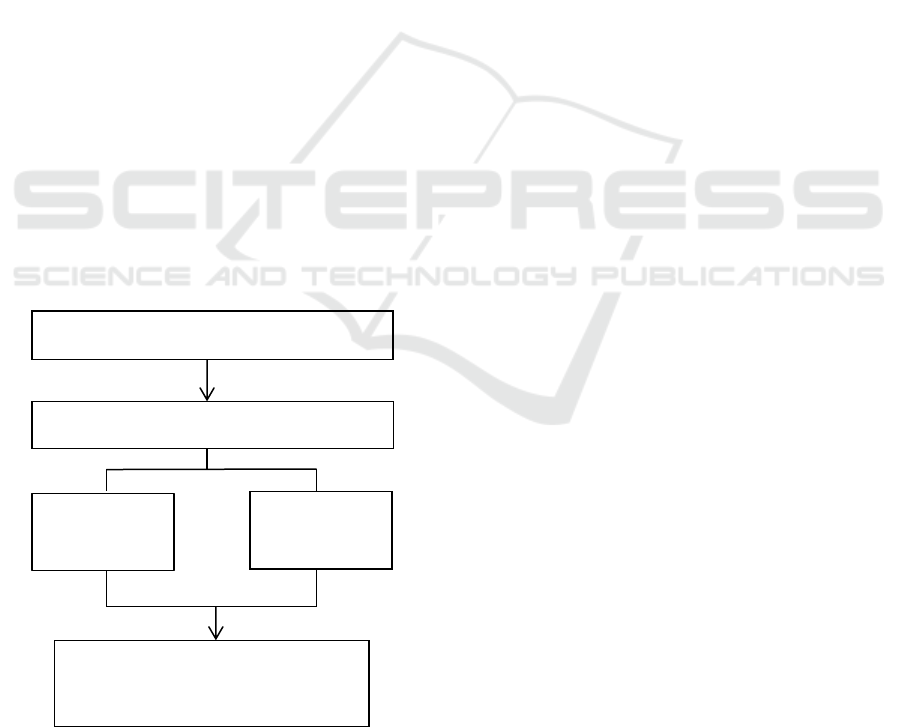
Figure 1: Flow chart of working plan.
This research was done by experiments, which
were directed to observe and explain a situation that
occurred (cause of effect) within certain time that
could not be controlled by researchers. The purpose
of this research was to measure the DNA tooth pulp
quantity exposure to seawater for 1 day and 7 days
based on concentration and purity of DNA for
forensic odontology.
This research used third molars tooth sample post-
retraction in healthy condition and without caries as
variables studied and DNA quantity as dependent
variables, and also the media of seawater and
exposure time as independent variables. The analysis
of total salt or salinity used the argentomtric method.
The seawater sample that were analyzed were filtered
first for remove impurities and then diluted.
Afterwards, argentometric titration had to be done.
2.2 Methods
2.2.1 Preparation of Third Molars Tooth
Samples
Third molar teeth exposed to seawater for 1 day and
7 days were drilled to obtain a tooth pulp portion
which was then dissolved into 300 μl aquadest in a
centrifuge tube. Then, they were vortexed
immediately, and the supernatant was removed and
then added with DNAzol 1000 μl (Invitrogen,
ThermoFisher Scientific, Waltham, MA, USA). The
solution was then vorteed again and incubated for 1
hour.
2.2.2 Extraction of Tooth Pulp DNA
The prepared sample was added with chloroform 200
μl (Merck KGaA, 64271 Darmstadt, Germany),
vortexed, and incubated overnight. After an overnight
incubation, the sample was vortexed for 2 minutes.
Then, the liquid of the sample was transferred to the
new centrifugation tube. The solution was centrifuged
at 8,000 rpm for 10 minutes. The supernatant was
taken carefully and fed into a 1.5 ml eppendrof tube,
which was then added with 70% isopropanol
(EMSURE*, Merck KGaA, 64271 Darmstadt,
Germany) until the tube was full and the contents
were homogenized. The process continued with
incubation for 30 minutes at room temperature. The
solution was centrifuged at 12,000 rpm for 10
minutes, then the supernatant was discarded. The
pellets were washed with 70% ethanol (EMSURE*,
Merck KGaA 64271 Darmstadt, Germany) by 1 ml,
and vortexed and incubated for one hour at room
temperature. The pellet was dried by means of
The quantity of DNA of tooth
pulp is the degree and purity of
DNA
7 days
immersion
time
Third molars Tooth after preparasion
Seawater exposure media
1 day
immersion
time
The Effect of Seawater on the Quantity of Dental Pulped DNA in Forensic Odontology Identification
441
opening the tube for 5-15 seconds after the 70%
ethanol was removed. This protocol followed Putri et
al (2016). It was followed by the addition of nuclease
fice water to the pellet of 50 μl as a DNA solvent,
which was vortexed and stored at -20 ° C to make
ready volume of DNA pellet for DNA quantification
with Ultraviolet-visible Spectrophotometer (UV-
1601, Shimadzu, Japan).
2.2.3 Measurement of Concentration and
Purity of Tooth Pulp DNA
Measurements were made using UV-VIS
instrumentation (UV-160, PC, Shimadzu, Japan),
where the eppendorf tube DW of 695 μl was added
with 5 μl isolated DNA, then vortexed. The
measurements of UV spectrophotometer absorbance
were conducted at wavelengths of 260 nm and 280
nm. This protocol followed Simon et al (2018), in
which DNA concentration was given by absorbance
reading at 260 nm and purity at 280 nm in UV-1061.
3 RESULTS AND DISCUSSION
Seawater media that was used came from the Straits
of Lombok, Indonesia, with 28.74% of total salt or
salinity. Salinity measurement was done by
argentometric method, which are the quantitative
analysis method of formation of sediment from salt.
The salinity of sea water media was still within the
normal range, because based on Praseno (2000)
research, the normal salinity range for tropical
seawater waters is 28 ‰ - 32 ‰.
The quantity of dental pulp DNA based on the
concentration of dental pulp DNA is as follows:
Table 1: Measurements of dental pulp DNA concentration.
Seawater
exposure
Absorbance
: 260 nm
DNA
Concentratio
n (ng/µl)
Average ±
SD DNA
concentrat
ion
(ng/µl)
1 day
0.577
0.535
0.562
1009.75
936.25
983.50
961.50 ±
41.53
After
(7 days)
0.454
0.490
0.423
794.50
857.50
740.25
797.42 ±
58.68
The results of measurements of dental DNA
pulp exposure to seawater for 1 day and 7 days
with a wavelength of 260 nm using UV
instrumentation yielded results in the form of
absorbances. The values of absorption or
absorbance obtained in the conversion in the
form of DNA content were determined by the
equation:
DNA Concentration = 260xFPx50ng/µl
The average ± SD dental DNA pulp
concentration 1-day exposure of sea water was
961.50 ± 41.53, while for 7 days it was 797.42
± 58.68. The decrease of DNA dental pulp
concentrations in this study indicated that there
was a structure of DNA dental pulp damage that
resulted in reduced DNA concentrations.
The quantities of dental pulp DNA based on the
purity of dental pulp DNA were as follows:
Table 2: Measurements result of tooth pulp purity
Seawater
exposure
Absorbance
Purity
of
DNA
Average
± SD
Purity of
DNA
260
nm
280
nm
1 day
0.577
0.535
0.562
0.549
0.521
0.547
1.05
1.03
1.03
1.04 ±
0.01
7 days
0.454
0.490
0.423
0.446
0.457
0.409
1.02
1.07
1.03
1.04 ±
0.03
The purity of dental pulp DNA exposure to
seawater for 1 day and 7 days with wavelengths of
260 nm and 280 nm using UV instrumentation
obtained results in the form of absorbance. The
value of absorption or absorbance obtained in the
conversion were in the form of DNA purity
obtained by the equation:
Purity of DNA = 260 : 280
The average value of ± SD DNA purity of dental
was stable at 1.04. This indicates that the selection
of DNA extraction was accurate and the next stage
could be conducted.
Third molars tooth pulp DNA isolation post
extraction was done by DNAzol method. The result
of tooth pulp DNA isolation was followed by
measurement of concentration and purity of DNA
using spectrophometer UV-vis at wavelengths of 260
nm and 280 nm (Putri, 2016). The measurement
function is to identify the tooth pulp DNA, since
measurement of concentration and purity of DNA
affected the success of next stage in DNA
identifications. Decrement of DNA concentration up
to 1 ng/µl potentially against decrement of detection
ICPS 2018 - 2nd International Conference Postgraduate School
442
ability of STR up to 95% (Putri, 2016). If the DNA in
bad degradation condition, it would cause the primer
to not attach to DNA target which would be
duplicated.
The measurement result of tooth pulp DNA
concentration and purity from third molar tooth
samples were shown in Tables 1 and 2. The tooth pulp
DNA concentration and purity seawater exposure for
1 day was 961.50 ng/µl and 1.04, while the tooth pulp
DNA concentration and purity for a seawater
exposure of 7 days were 797.42 ng/µl and 1.04; The
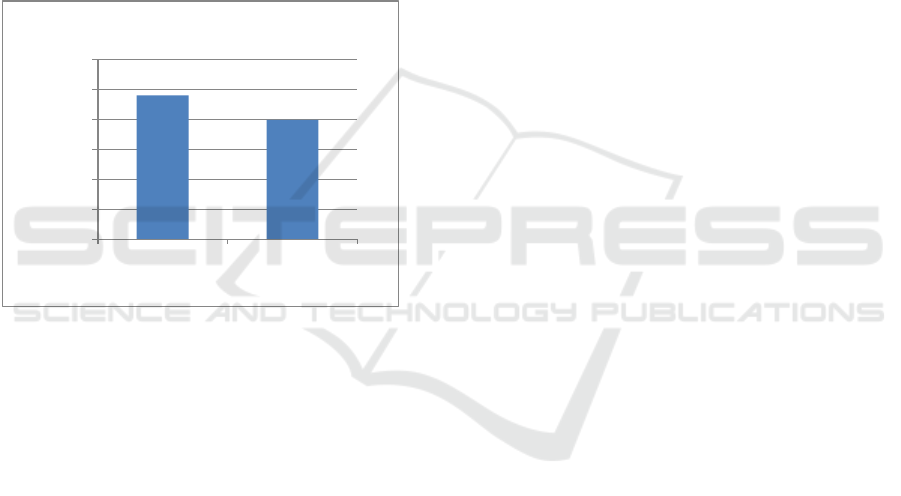
graphic below (Figure 2) shows the decrement of
concentration after exposure seawater media. There
was decrement of tooth pulp DNA concentration in
this study, showing that the broken structure of tooth
pulp DNA caused the decrement of DNA
concentration.
Figure 2: Graphic of decrement of tooth pulp DNA
concentration
In groups of teeth, seawater exposure showed
conformity concentration of tooth pulp DNA
concentration and purity of 961.50 ng/µl dan 1.04.
Irnamanda (2016) found that tooth pulp had high
conformity concentration with post-extraction of
tooth sample within 6 months (180 days) without
treatment. Since morphology is composed by the
hardest enamel substance and dentine that protects
tooth pulp. Therefore, the pulp can protect DNA and
experienced slow postmortem changes. However,
after the exposure of seawater media treatment was
done, there were degradations of DNA concentration.
This is caused by enamel composition consisting
mostly of hydroxyapatite salt, soluble materials
(mucopolysaccharide), and insoluble substance
(keratin), which could easily absorb water, causing
the enamel to become semipermeable (penetrating by
water). The diffusion path gap between crystal
arrangement caused the enamel to be said as
microporous solid material. The gap between apatite
crystals containing the organic and water matrix
occurred due to the structure of the hexagonal
hydroxyapatite crystals that caused a perfect bond to
be difficult to make.
Enamel is composed by inorganic substances and
organics substance, but they do not bind together
because of the hexagonal crystal structure, thus the
lead gap between apatite crystal arrangements
(Manjunatha, 2013).
Seawater is liquid, which means that it can
penetrate the enamel and dentin due to a gap between
the apatite crystal structure, since seawater, which is
used for soaking tooth, cause liquid outside cells to
diffuse into cells due to potential differences of sea
water and red blood cells (Irnamanda, 2016).
Most salt-containing seawater has a higher
potential than red blood cells. Therefore, sea water
enters erythrocytes and causes the erythrocyte
membrane to rupture and erythrocyte cytoplasm to
exit. The higher the osmotic pressure, the easier the
red blood cells in the tooth are pulled out because of
the semipermeable cell membrane of water. The teeth
exposed to sea water can affect the structure of DNA
contained in the dental pulp (Irnamanda, 2016).
Damages to the structure of DNA caused by exposure
to seawater media result in the destruction of
hydrogen DNA bonds that are irreversible. One of
them is on the damage of base pairs purine-
pyrimidine in DNA, which is the main component in
the structure of DNA (Putri, 2016).
961,5
797,42
0
200
400
600
800
1000
1200
Sebelum Sesudah
DNA Level
Exposure Seawater Media
Concentration of Tooth Pulp DNA (ng/µl)
1 day 7 days
The Effect of Seawater on the Quantity of Dental Pulped DNA in Forensic Odontology Identification
443
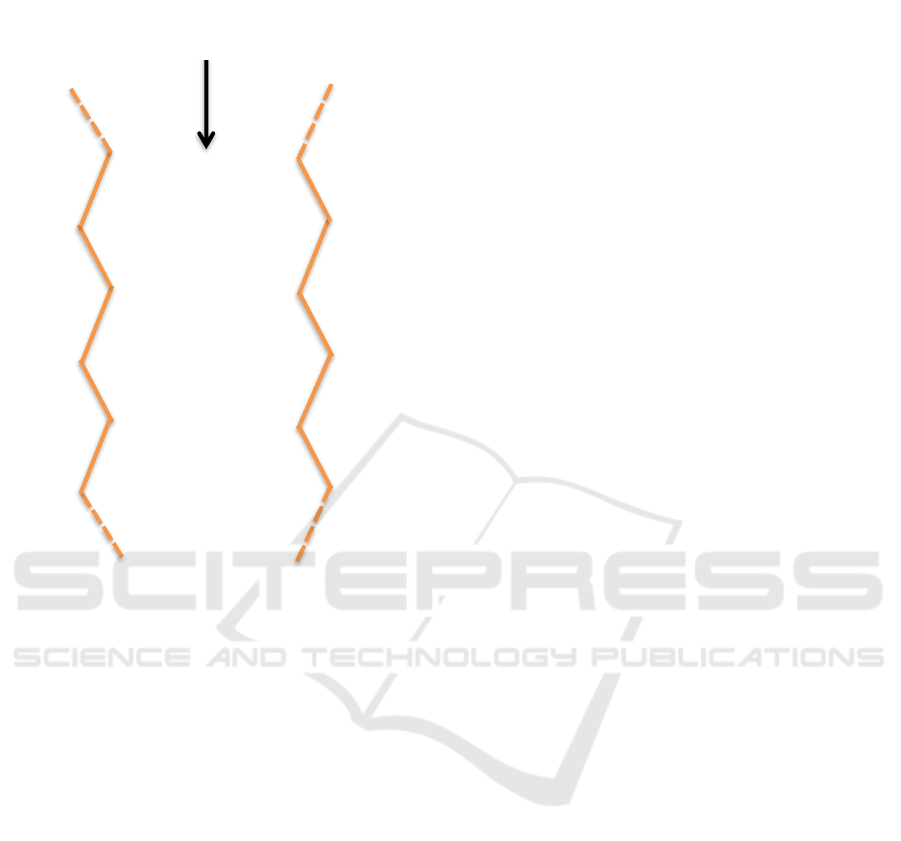
Figure 3: DNA structure of the purine-pyrimidine nitrogen
base pair
The osmotic pressure on seawater is related to the
salinity of seawater. The greater the salinity in sea
water, the higher the osmotic pressure. In this study,
the sea water used comes from the Strait of Lombok
with total salt or salinity of 28.74‰. Salinity
measurement was done by argentometric method, that
is quantitative analysis method of formation of
sediment from salt. The salinity of sea water media
was still within the normal range, because based on
Praseno (2000), the normal salinity range for tropical
seawater waters is 28‰ - 32‰.
4 CONCLUSION
The quantity of dental pulp DNA processing in
forensic DNA profiling, together with quality
(purity), are important parameters for human
identification. This study determined the
effectiveness of seawater to the quantity of teeth
pulped DNA by observing and presenting the trend.
The factor’s (sea water) exposure yielded an effect on
DNA quantity for day 1 and 7, in which the amount
of DNA decreased. This acts as an evidence that sea
water has a potentially interfering and damaging
outcome when targeted sample is found to be in
contact. Effectiveness is suggested by high
concentration of salinity contained in sea water,
though it did not establish the minimal concentration
of salinity. This study therefore concluded the
existence of an impending effect from exposure to
seawater. In addition, this study also significantly
contributes to the understanding of the sea water
medium in prediction of DNA quantity that could be
expected at the prescribed duration of PMI.
Furthermore, the finding triggers discussion towards
experimentation of the minimal benchmark values of
sea water salinity significance.
ACKNOWLEDGEMENTS
The authors would like to thank the technicians of the
Human Genetic of Tropical Diseases (ITD)
Laboratory of Airlangga University and all those who
have assisted in the completion of this research.
REFERENCES
Datta, P., 2012. DNA Profiling in Forensic Dentistry. J
Indian Acad Forensic Med. April-June 2012, Vol. 34,
No. 2.
Febri, K.A., 2013. Heteroplasmy Analysis of Dental Pulp
Mitochondrial DNA in Forensic Personal
Identification. Dental Journal Vol. 46, Number 3,
September 2013.
Girish, JK., 2010. Dental DNA Fingerprinting in
Identification of Human Remains. J Journal Forensic
Dent Sci. 2010. Jul-Dec 2(2): 63-68.
Irnamanda, D.H., 2016. Effect of Seawater on ABO Blood
Type Determination Accuracy of Dental Pulp. KONAS
PDFI 2016.
Krishan, K., 2015. Dental Evidence in Forensic
Identification-An Overview, Methodology and Present
Status. The Open Dentistry Journal, 2015, 9, 250-256.
Nzilibili, S.M.M et al, 2018. Concentration and Purity
DNA Spectrophotometer: Sodium Monofluoro
phosphate Forensic Impended Effect. Egyptian Journal
of Forensic Science 8:34.
Manjunatha BS, 2013. Dental Anatomy and Oral
Physiology Including Occlusion and Forensic
Odontology. Jaypee Brothers Medical Publishers (P)
LTD: New Delhi, India.
Putri, N.P and Yudianto A., 2016. Effect Of Soil and Sea
Water to Quality Of Psoas Muscle DNA Corpse With
STR Methode. Journal Bioscience Pascasarjana Vo. 18
(2016) No. 3, December 2016.
Prasero, D.P., 2000. Retaid di Perairan Indonesia. LIPI.
Jakarta: Hal 82.
Sugar-Base......Base-Sugar
Sugar-Base......Base-Sugar
Sugar-Base......Base-
Sugar
Phosphat
e
Phosphate
Phosphate
Phosphat
e
Phosphat
e
Phosphat
e
Hydrogen Bonds
ICPS 2018 - 2nd International Conference Postgraduate School
444

Rai, B., 2012. DNA Technology and Forensic Odontology.
Spinger Link: Evidence Based Forensic Dentistry.
The Effect of Seawater on the Quantity of Dental Pulped DNA in Forensic Odontology Identification
445
