
Optimization of Nano Coral-Based Synthesis Calcium Phosphate with
Concentration Variation of Phosphoric Acid and Sintering
Temperature
Siswanto, Mayasari Hariyanto and Siti Nurmala
Department of Physics, Faculty of Science and Technology, Universitas Airlangga, Surabaya, Indonesia
Keywords: Calcium Phosphate, Coral, Phosphoric Acid Concentration, Sintering Temperature
Abstract: Research on the optimization of calcium phosphate formation has been carried out. The material used is
nanocoral with varying concentrations of phosphoric acid and sintering temperature. The method used is
precipitation method. There were three variations of molar concentration of phosphoric acid used, namely
1M; 1.5M; and 2M. While the variations in sintering temperature were 800ᵒC, 850ᵒC, 900ᵒC and 950ᵒC
respectively. Nano-sized powders were obtained through mechano-chemical processes of fossilized corals
using High Energy Milling (HEM) for 20 hours, which is 64.93nm. X-Ray Diffraction (XRD) observation
results showed that the Calcium Phosphate phase formed is Hydroxyapatite (HAp), β-Tricalcium Phosphate
(β-TCP) and Tetra Calcium Phosphate (TTCP). The TTCP phase only occurred at 950
0
C. The largest
volume of HAp fraction occurred at the molar concentration of 1.5 M of phosphoric acid with sintering
temperature of 850ᵒC, which is 70.7% and the remaining β-TCP phase is 29.2%. The crystallinity
percentage that occurred in these conditions is 94.95%.
1 INTRODUCTION
The Indonesian Ministry of Health Research and
Development Agency (RISKERDAS, 2013), stated
that there was approximately 5.8% of fractured
injury sufferers in Indonesia. This figure increased
by 0.7% compared to the same incident in 2007.
Those injury cases are caused by various factors
including traffic accidents, natural disasters and
bone cancerous.
Improvements to bone injury can be done by
using filler, scafold or implant. Until now, 90% of
the material is still imported. Someone who has bone
damage spends about 70% of treatment cost for
implants. This fact shows the importance of
independence in providing medical materials
including calcium phosphate biomaterials.
Calcium phosphate is the main mineral
constituent of bones and teeth. Calcium phosphate
can induce a biological response during bone
renewal or formation by performing bone mineral
absorption. When absorption occurs, the results of
calcium phosphate degradation (calcium and
phosphate ions) will be metabolized by the body
naturally. In general, human bones consist of 60%
inorganic ingredients, 30% organic matter, and 15%
water (Krishna et. al., 2007). Inorganic material is
bone mineral containing sub-microscopic
Hydroxyapatite [Ca
10
(PO
4
)
6
(OH)
2
]. Other inorganic
minerals are magnesium (Mg), fluoride (F), Chloride
(Cl), sodium (Na) and potassium (K). There is about
34.00% of calcium (Ca) compounds and 15% of
phosphorus (P) compounds in human bones (Darwis,
D. & Warastuti, Y., 2008). Calcium phosphate can
be obtained from the synthesis of natural materials
with high calcium, one of which is coral.
Research on coral rock used for raw materials
implant has been carried out by previous
researchers. A research conducted by Indarwati
(2007) on coral rock analysis using XRD (X-Ray
Diffraction) test showed that the composition of the
coral is 91.696% CaCO
3
(Aragonite) compound,
3.677% MgSiO
3
, and 4.626% FeSi. From the results
of the analysis, it appears that the coral contains very
high calcium carbonate (CaCO3) compounds.
Calcium carbonate compounds (CaCO
3
) can be
converted into HAp through various processes. One
of them is through the calcination process to form
CaO and Ca(OH)
2
compounds. Furthermore,
calcium phosphate compounds can be formed by
Siswanto, ., Hariyanto, M. and Nurmala, S.
Optimization of Nano Coral-Based Synthesis Calcium Phosphate with Concentration Variation of Phosphoric Acid and Sintering Temperature.
DOI: 10.5220/0007545104650468
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 465-468
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
465
reacting them with phosphoric acid which is sintered
at a certain temperature.
Therefore, this study focused on the formation
of calcium phosphate by optimizing the process
parameters of phosphoric acid’s molar concentration
and sintering temperature variations. This is based
on the fact that coral is a natural mineral that
contains many compounds so that the right molar
concentration of phosphoric acid is needed. In
addition, the formation process also requires the
right sintering temperature. To accelerate the
reaction process of calcium phosphate formation in
this study, the expansion of surface area of the touch
reaction through the size of nanoparticles was
carried out. The smaller the particle size, the wider
the surface area of the touch (Ferraz, 2014).
2 MATERIAL AND METHODS
Coral used in this study came from the sea in
Banyuwangi, East Java, Indonesia. Phosphoric acid
(H
3
PO
4
) with a purity of 99.8% Aldrick, aquadest
and glycerol.
This research was done through two stages of
nano coral and calcium phosphate formation. The
first stage was the formation of nano coral using top
down method, which converts large particles into
nano-sized particles through the milling process. The
corals were cleaned from dirt and dried in the open
space. Then, the coral was destroyed manually to be
smaller particles. Once becoming sufficiently
smooth, the sample was sieved using a 200 mesh
sieve to facilitate the milling process. Then, the
result of the sieve was milled using HEM-3D (High
Energy Milling-3 Dimension) for 20 hours with
comparison between samples of 1:20 to ball mill.
The formation of calcium phosphate based on
nano coral was performed using precipitation
method. The molarity of phosphoric acid used varies
i.e. 1.0M; 1.5M; 2.0M, while the sintering
temperature was carried out at four different
temperatures i.e. 800
0
C; 850
0
C; 900
0
C; and 950
0
C.
So, there were 12 samples used in this research.
3 RESULT AND DISCUSSION
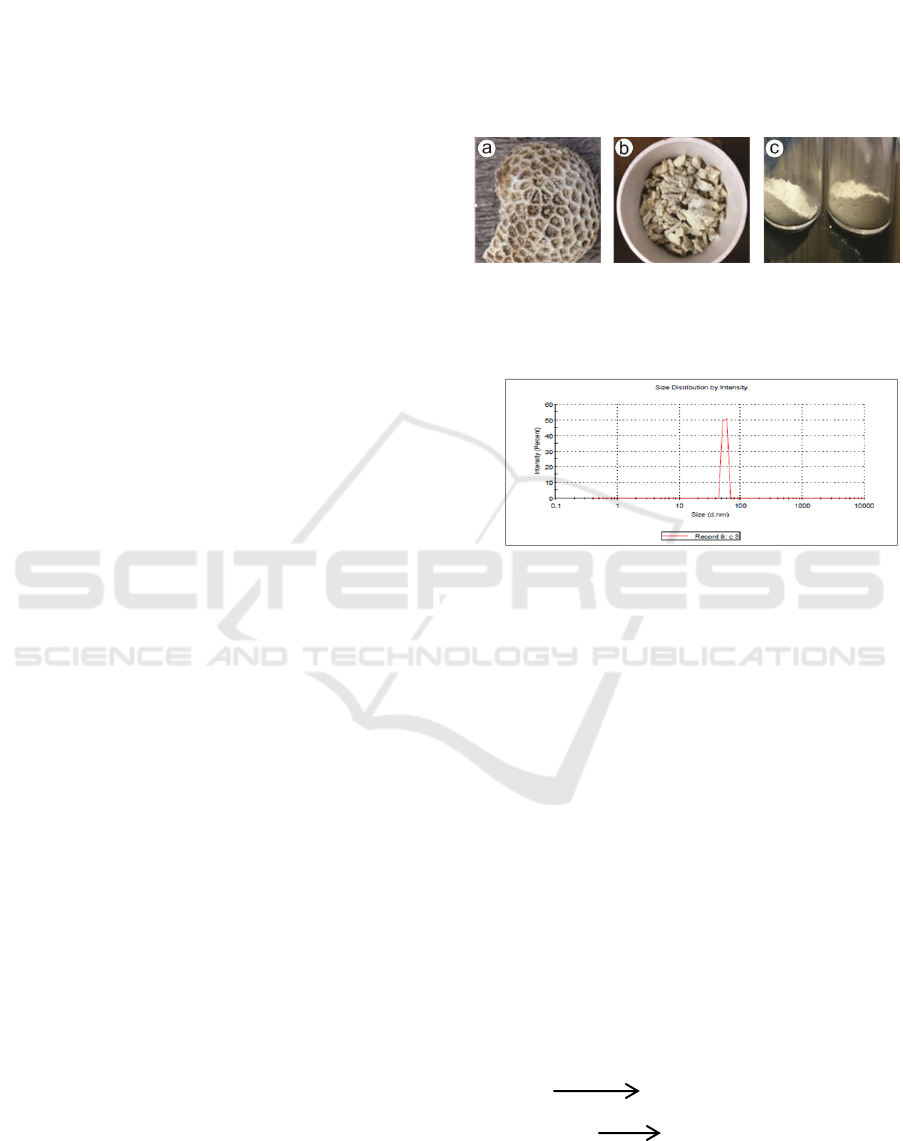
The corals used in this study came from the
Banyuwangi sea of East Java (figure 1a). The type
of coral is fossilized coral. The coral was then
broken into small sizes using a hammer (Figure 1b).
To facilitate the milling process, the results of the
coral pieces were crushed using a mortar then
filtered using a 200 mesh sieve (Figure 1c). The
results of the filter were milled using HEM-3D for
20 hours to change the size of the nanoparticles. The
results of the observation of Particle Size Analyzer
(PSA) on the results of the treatment showed that the
size obtained was 64.93 nm (Figure 2).
Figure 1: The process of sample preparation (a) coral has
been cleaned, (b) coral has been dissolved, (c) coral has
been refined.
Figure 2: The results of PSA test of coral powder
Nano coral XRD diffractogram was done to
determine the content of the compound. Figure 3
shows a sample of nano coral diffractogram. By
using the Search-Match program (see Figure 3), it
can be found that the coral consists of 78.6% CaCO
3
(calcite) compounds and 21.4% Ca
2
O
5
Si
compounds. The CaCO
3
calcination process causes
the change of the compound to CaO and CO
2
as
shown in Equation 1. Furthermore, the milling
process of calcined coral can cause a thermal
reaction that produces water vapor. In the vial, the
moisture formed can react with CaO which produces
Ca(OH)
2
, as stated in Equation (2). This can be seen
in Figure 4 diffractogram, the coral produced 95.8%
Ca(OH)
2
and 4.2% CaO. The presence of CaO is
still suspected because the reaction process in the
vial is still less than optimal. This can be caused by
short time for milling and improper comparison of
ball vials.
CaCO
3
900 ºC
CaO + CO
2
……………(1)
CaO + H
2
O Ca(OH)
2
……….. …...(2)
ICPS 2018 - 2nd International Conference Postgraduate School
466
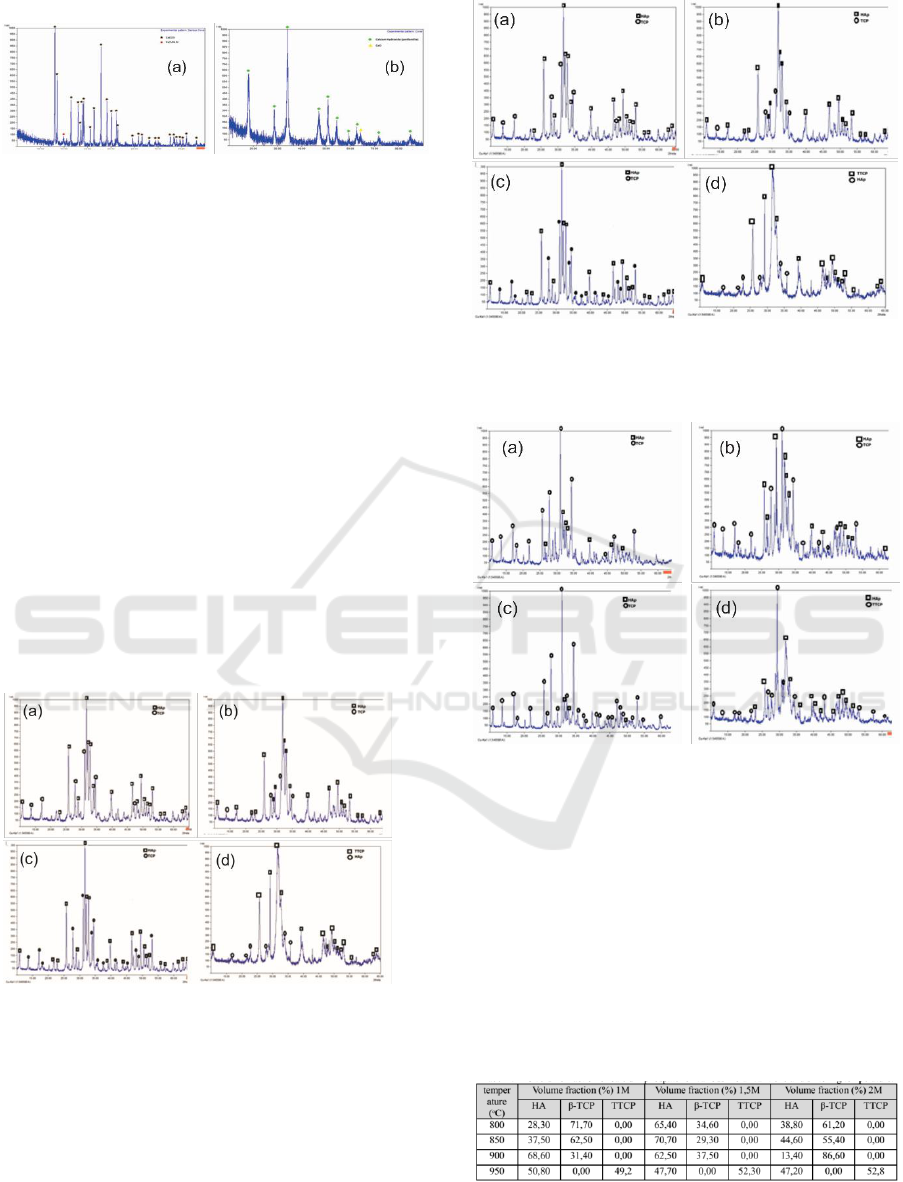
Figure 3: The results of XRD observation (a) nano coral
(b) nano coral calcined at 900
0
C
Calcium phosphate was formed by the reaction
of calcium hydroxide (Ca(OH)
2
) and phosphoric
acid (H
3
PO
4
), as shown in Equation (3). The
treatment of phosphorus acid molar variation was
done because the results of XRD (see Figure 4) still
contained CaO as impurity form of Ca(OH)
2
. In this
study, there were 3 variations of phosphoric acid
molarity used i.e. 1M, 1.5M and 2M. In addition, the
process of ceramic formation generally is strongly
influenced by the sintering temperature. In this
study, four variations of sintering temperature were
carried out. Therefore, every variation of molar
above was performed by four variations of sintering
temperature which are 800
0
C; 850
0
C; 900
0
C; and
950
0
C, so there were total of 12 samples used in this
study. The results of observations of 12 samples are
displayed in Figure 5, Figure 6, and Figure 7.
5Ca(OH)
2
+3H
3
PO
4
→Ca
5
(PO
4
)
3
(OH) +9H
2
O (3)
Figure 4: XRD observations of 1 M calcium phosphate
sample with sintering temperature at
(a) 800
0
C, (b) 850
0
C, (c) 900
0
C, and (d) 950
0
C
Figure 5: XRD observations of 1.5M calcium phosphate
sample with sintering temperature at (a) 800
0
C, (b) 850
0
C,
(c) 900
0
C, and (d) 950
0
C
Figure 6: XRD observations of 1 M calcium phosphate
sample with sintering temperature at (a) 800
0
C, (b) 850
0
C,
(c) 900
0
C, and (d) 950
0
C
The identification of the XRD in Figure 4, Figure
5, and Figure 6 shows that the calcium phosphate
formed is the HAp (Hydroxyapatite), TCP (Tri
Calcium Phosphate), and TTCP (Tetra Calcium
Phosphate) phases. In full, the volume fraction from
these three phases for various molar concentration
and sintering temperature are shown in Table 1.
Tabel 1: The volume fraction of calcium phosphate for
various molar concentration and sintering temperature
From Table 1, it appears that the stable calcium
phosphate phase, Hap, is highly dependent on
molarity of phosphoric acid and sintering
Optimization of Nano Coral-Based Synthesis Calcium Phosphate with Concentration Variation of Phosphoric Acid and Sintering
Temperature
467
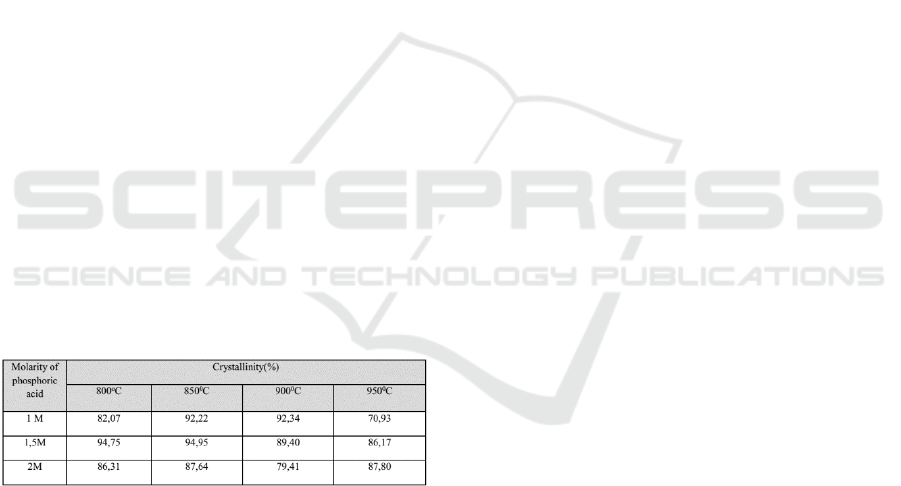
temperature. This optimum phase occurred at a
sintering temperature of 900
0
C with molarity of 1M
phosphoric acid. In addition, this phase is also
optimized at 850
0
C sintering temperature with 1.5M
phosphoric acid molarity. This also occurred with
the crystallinity formed, as stated in Table 2.
Human bone crystallinity is in the range of 69%
to 87% (Balgies, 2011), while the dominant phase of
calcium phosphate in bone is HAp with a Ca / P
ratio of 1.67. The Ca / P ratio of β-TCP and TTCP
compounds are 1.50 and 2.0, respectively (Kerry L.
and Hull, P., 2011). Based on these references, there
are five crystallinity samples that fulfil the
requirements as bone filler. Based on the heating
process, 950
0
C sintering temperature is the most
effective compared to other sintering temperatures.
Based on its molarity, the 2M molar concentration of
phosphoric acid is the most effective. This is
allegedly caused by chemical reactions that occur
between CaO and H
2
O is less than perfect, so that
Ca(OH)
2
is formed and there is still residual CaO
(Sokolova,2012). The number of HAp phases, β-
TCP phase and TTCP phase affect the Ca / P ratio of
the material. The ratio of the three phases in a row is
1.67, 1.50 and 2.00. By using the mean principle
from Table 1, the ratio of Ca / P of the sample can
be determined. When viewed from the molarity of
phosphoric acid, the 1.5M molarity has the best
value of 1.67. However, when viewed from the
sintering temperature, 900
0
C is the most optimal
temperature, which gives a Ca / P ratio of 1.61
Tabel 2 : Crystallinity of Hydroxyapatite
4 CONCLUSIONS
From the series of analysis and discussion that
has been done, some conclusions can be drawn.
First, the sintering process causes the transformation
of the structure phase from amorphous to crystal.
The crystallinity of calcium phosphate is influenced
by the sintering temperature and the molar
concentration of phosphoric acid. The sintering
temperature of 800
0
C and 850
0
C give the optimum
crystallinity. Second, the mole amount of phosphoric
acid greatly influences both the phase and
crystallinity. Finally, the stable phase of HAp was
optimally formed at 850
0
C with 1.5M phosphate
molarity of both volume fraction and crystallinity.
ACKNOWLEDGEMENTS
The authors would like to thank the Ministries of
Research, Technology, and Higher Education for its
support through the Exceptional Applied Research
in Higher Education program (Penelitian Terapan
Unggulan Perguruan Tinggi or PTUPT), 2018 fiscal
year.
REFERENCES
Balgies. (2011). Synthesis and Characterization of
Hydroxyapatite from Ranga Shellfish Shells
Final Project of Physics Program Study,
IPB (Institut Pertanian Bogor).
Darwis, D., & Warastuti, Y. (2008). Synthesis and
Characterization Composite of
Hydroxyapatite (HA) for synthetic Bone
Graft, Jurnal Ilmiah Aplikasi Isotop dan
Radiasi, 4 No.2.
Ferraz, M. P., J., M. F., & M., M. C. (2014).
Hydroxyapatite Nanoparticles: A Review of
Preparation Methodologies (Vol. 2). J.
Appl. Biomaterials Biomechanics.
Indarwati, D. (2007). Analysis of Coral as Implant
Material, Final Project of Physics Program
Sudy, University of Airlangga , Surabaya.
Kerry L. and Hull, P. (2011). Study Guide to
Accompany Human Form, Human Function
Essentials of Anatomy & Physiology (Vol.
I). Sherbrooke, Quebec, Canada: Lippincott
Williams & Wilkins, a Wolters Kluwer
business.
Krishna, D. S. R. A. Siddharthan, S. K.Seshadri, T.
S. S. Kumar, 2007, A Novel Route for
Synthesis of Nanocrystalline
Hydroxyapatite FromEggshell Waste.
Material Medicine,Vol 18, p. 1735-1743
Sokolova, M., Kreicbergs, I., Zalite, V., & Berzina-
Cimdina, L. (2012). The impact of Ca(OH)
2
suspension concentration on hydroxyapatite
synthesis. Proceedings of the Eurasia 12
Conference on Chemical Science, Corfu,
Greece, 16-21 April
ICPS 2018 - 2nd International Conference Postgraduate School
468
