
Leukocyte Count and Differential Leukocyte Count of Carp
(Cyprinus carpio Linn) after Infected by Aeromonas salmonicida
Abdika Dwi Afiyanti
1
, M.Gandul Atik Yuliani
1
and Didik Handijatno
2
1
Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia
2
Department of Microbiology, Universitas Airlangga, Surabaya, Indonesia
Keywords: Carp, Aeromonas salmonicida, total leukocyte, differential counting leukocyte.
Abstract: The hematology examination is to describe the health of fish. The aim of this research is to study leukocyte
and differential leukocyte count in Carp (Cyprinus carpio Linn) after infected by Aeromonas salmonicida. P0
was a control group and waa not infected with Aeromonas salmonicida. P1, P2, and P3 group were infected
by Aeromonas salmonicida through different doses; 105 cell/ml, 106 cell/ml, and 107 cell/ml. Three days
after infection, blood samples were obtained from Punctie Vena Caudal which was deposited into EDTA
tubes. Total number leukocyte and differential leukocyte count analyzed were carried out with the collected
blood samples. Data were analyzed with ANOVA (Analysis of Variant) then followed by Duncan’s multiple
range test significance level of 5%. The results showed that there was significant increase (p<0.05) in the
number of leukocytes compared with control (P0). There was significant increase (p<0.05 ) in the number of
eosinophils, neutrophils, lymphocytes and monocytes. The number of basophils were not significant (p<0.05).
Based on the results of research, it could be concluded that bacterial infections Aeromonas salmonicida in
Carp cause changes in leukocyte count and differential leukocyte count. The change is an increase in the
number of leukocytes count; while in the differential leukocyte count, there was an increase in eosinophils,
neutrophils, lymphocytes and monocytes.
1 INTRODUCTION
Indonesia has abundant freshwater and is
considerable potential for the cultivation of a wide
variety of freshwater fish species (Cahyono, 2000).
Freshwater fish have a relatively high protein. High
content of protein and vitamins cause freshwater fish
be easily cultivated and very helpful in nutrition
fulfillment for the society. This is due to the fact that
fish is an excellent source of protein, fat, and minerals
(Ministry of Maritime Affairs and Fisheries, 2014).
Carp is considered as the most popular freshwater
fish among the existing freshwater fish species
(Supriatna, 2013). Various cultivation systems have
been applied and prolonged to grow to obtain maximum
goldfish production. One of the cultivation is by
applying intensive cultivation system. However, the
intensive Carp farming also has a negative impact, one
of which is susceptible fish disease. One of the
dangerous diseases is caused by Aeromonas bacterial
infections; such as Aeromonas salmonicida. Aeromonas
salmonicida is a cause of infectious diseases in salmonid
fish that is furunkulosis disease (Nitimulyo et al., 1993).
A number of reports indicate that there are also
symptoms of bacterial infection Aeromonas salmonicida
in cyprinid fish, namely carp erytrodermatitis disease
(Irianto, 2005).
The presence of bacterial infections Aeromonas
salmonicida characterized by changes in various
clinical symptoms affects the blood picture of Carp.
Blood picture is one indicator of infection (Nuryati et
al., 2006). In the field of fisheries, hematological
analysis can be applied as an early detection system
to prevent mass death in fish cultivation (Noercholis
et al., 2013).
Research on the number and counts of leukocyte of
certain species can provide an overview of the
environmental state of the fish, providing information
about the health status and process of the occurrence of
a disease in it. By analyzing the blood characteristics,
a disease can be identified (Kumar and Ramulu,
2013). This is what encourages researchers to do
research on the examination of and calculate the
number and the type of Carp (Cyprinus carpio Linn)
blood leucocytes after infected by Aeromonas
salmonicida.
Afiyanti, A., Yuliani, M. and Handijatno, D.
Leukocyte Count and Differential Leukocyte Count of Carp (Cyprinus carpio Linn) after Infected by Aeromonas salmonicida.
DOI: 10.5220/0007546705450549
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 545-549
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
545

2 MATERIALS AND METHODS
2.1 Place and Time of Research
This research was conducted in October - December
2017. The process of fish maintenance was done in
the Laboratory of Veterinary Pathology, Department
of Veterinary Pathology. The process of isolation and
dilution of bacteria Aeromonas salmonicida was
executed in the Laboratory of Bacteriology and
Mycology, Department of Veterinary Microbiology.
The process of taking blood, making blood smear,
and examination of leukocyte count and differential
leukocyte count was applied at Veterinary Clinical
Pathology Laboratory, Departement of Veterinary
Basic Medicine, Faculty of Veterinary Medicine,
Airlangga University.
2.2 Material and Equipment of Research
The materials used in this research were Carp, pellet
fish feed, water taps, Triple Soy Agar (TSA) Media,
Triple Sugar Iron Agar (TSIA) Media, Sulfide Indol
Motility (SIM) Media, Simon Citrate Agar (SCA)
Media, Urea and MR-VP Media, physiologic
NaCl, bacterial isolates Aeromonas salmonicida,
EDTA solution, Dacies solution, 95% methanol,
Giemsa dye and Giemsa buffer solution and oil
emersion. The equipment used in this research were
four aquariums containing 20 liters water, aerator
machine, aerator hose, water purifier filter, zeolite
stone, digital milligram scales, small fish net, pH
meter, DO meter, 0.1 ml pipette with scale 0.001 ml,
ose, petri dish, test tube, bunsen, petri dish, disposible
syringe with needle, Improved Neubauer count
chamber, Pasteur pipette, Blood Cell Counter, glass
cover, light microscope, object glass, and glassware
shelf.
2.3 Methods of Research
This study used Carp (Cyprinus carpio Linn) with
length10-12 cm (age 6-9 weeks) and weight 21-23 g.
Of 20 fish samples were randomized to four
treatments, each treatment consisted of five
replications. Carp adapted for one week.
2.4 Research Design
The experimental design used was Completely
Randomized Design (RAL). The experiment was
conducted with four treatments and five repetitions.
Treatment under study:
P0: Negative control treatment group. The first
aquarium was not infected by Aeromonas
salmonicida
P1: Second aquarium was infected by Aeromonas
salmonicida with a dose of 10
5
cells / ml P2: Third
aquarium was infected by Aeromonas salmonicida
with a dose of 10
6
cells / ml P3: Fourth aquarium
was infected by Aeromonas salmonicida with dose
10
7
cells / ml
2.5 Data Processing
The data obtained in the form of the leukocyte count
and differential leukocyte count were arranged in
tabular form and then analyzed. Furthermore, a
statistical test was performed using ANOVA
(Analysis of Variant). If there was any difference
between treatments proceed and Duncan Multiple
Range Test, it would be a significance level of 5% to
determine the best treatment. Data analysis was
performed using SPSS 20 for Windows computer
software.
3 RESULTS AND DISCUSSION
3.1 Leukocyte Count
The results of the total count of leukocytes in goldfish
after given the treatment in detail can be seen in the
following table:
Table 1: Mean and Standard Deviation of Carp Leukocyte
After Infected by Aeromonas salmonicida.
Treatment
Leukocytes
(cell/mm
3
) (X ± SD)
P0 (without
12400,00
a
± 1013,66
infection)
19210,00
b
± 2278,54
P1 (infection with
dose 10
5
cell/ml)
P2 (infection with
25990,00
c
± 3813,37
dose 10
6
cell/ml)
45270,00
d
± 6986,65
P3 (infection with
dose 10
7
cell/ml)
Description: Different superscript letters in the same
column show significantly different (p<0,05).
ICPS 2018 - 2nd International Conference Postgraduate School
546
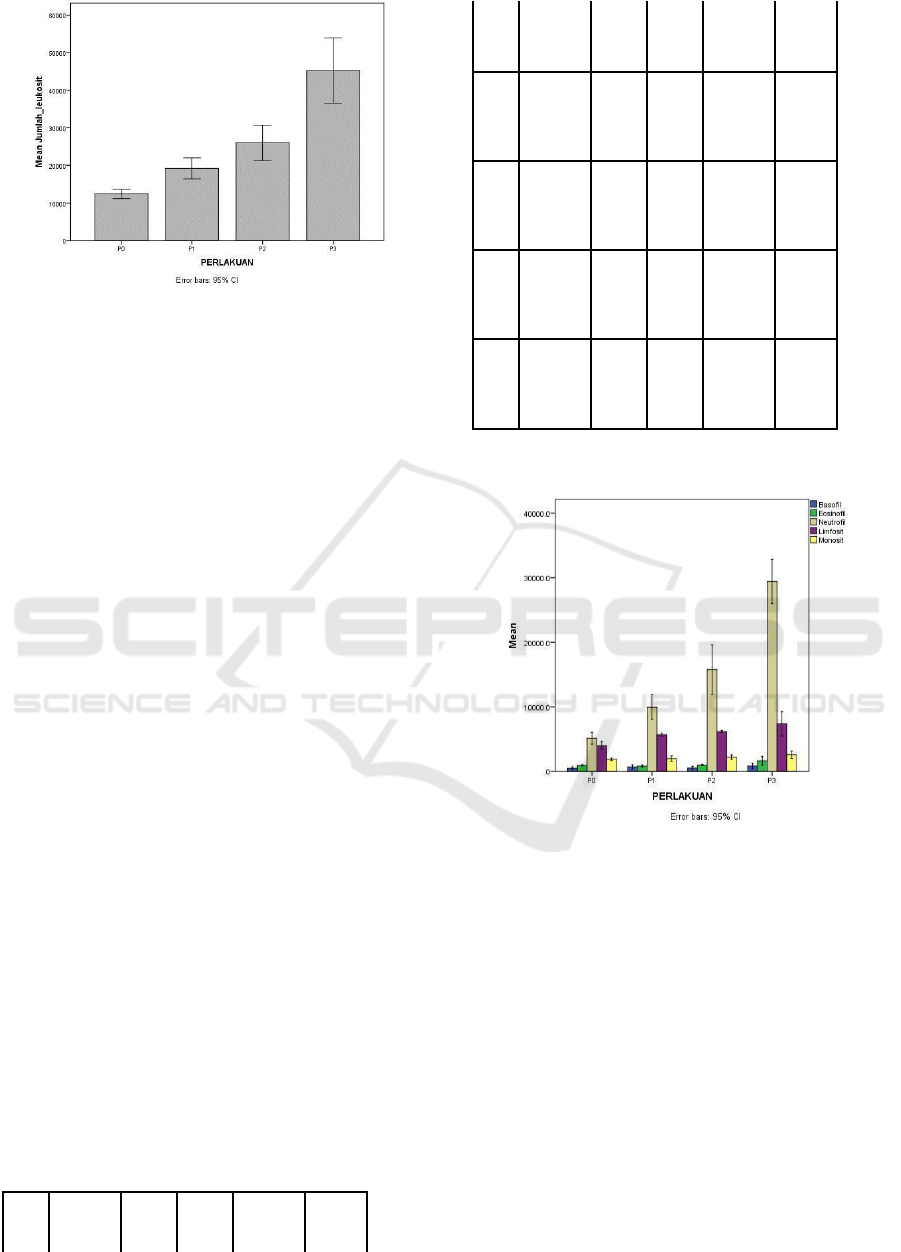
Figure 1: Graph of Leukocytes of Carp After Infected by
Aeromonas salmonicida.
The results after analyzed by ANOVA assay
showed that there was a significant difference in the
number of leukocytes among treatments. After tested
with Duncan Multiple Range Test, results illustrated
an increase in the number of leukocytes were
significantly different (p <0.05). P0 was significantly
different from P1, P2, and P3, while P1 was noticably
different with P2 and P3.
Increased number of leukocyte cells in goldfish after
treatment showed an immune response to the infection
of Aeromonas salmonicida. This is in accordance with
the opinion of Alamanda et al. (2007), which states that
the increase in total leukocytes indicates a response of
body resistance to disease-causing antigens. Increased
total leukocytes showed an increase in body immunity
characterized by increased activity of phagocyte cells
that function to perform phagocytosis against foreign
objects entering the fish body. Leukocyte systems and
tissue cells of leukocytes work in two ways to prevent
disease by damaging through the process of
phagocytosis and forming antibodies (Suhermanto,
2013). Phagocytosis is the first step for mechanism of
immune response, the next is the formation of specific
antibody responses, whereas the increased phagocytic
process suggests an increase in body immunity (Zainun,
2007).
3.2 Differential Leukocyte Count
The results of the leukocyte count in carp after
treatment were given as follows:
Table 2: Mean and Standard Deviation of Differential
Leukocyte of Carp after infected by Aeromonas
salmonicida.
Tr
Eosino
Baso
Neut
Lymph
Mono
eat
me
phil
phil
rophi
ocyte
cyte
nt
(X±
(X ±
l
(X±
(X ±
SD)
SD
(X ±
SD)
SD)
SD)
P0
934,50
a
499,
5121
3972,9
1871,
± 88,21
70
a
±
,00
a
±
0
a
±525,
90
a
±1
171,
736,
08
54,13
21
72
P1
824,75
a
529,
9947
5662,1
1967,
±157,3
36
a
±
,30
b
±
0
b
±205
30
a
±3
8
265,
1557
,02
49,46
96
,88
P2
982,75
a
705,
1573
6148,2
2215,
± 75,31
30
a
±
8,30
c
5
b
±146
40
ab
±
191,
±312
,70
285,9
70
0,51
0
P3
1634,8
849,
2941
7359,1
2582,
0
b
±582
45
a
±
5,00
d
0
c
±153
600
b
±
,15
348,
±274
6,28
415,2
33
3,15
0
Description: Different superscript letters in the same
column show significantly different (p <0.05).
Figure 2. Graph of Differential Leukocytes of Carp After
Infected by Aeromonas salmonicida.
The results after analyzed by ANOVA assay showed
that there were significant differences in the number of
neutrophils, eosinophils, lymphocytes and monocytes
among treatments. After tested with Duncan Multiple
Duncan Test, there was a noticable
increase of eosinophil, neutrophil, lymphocyte and
monocyte counts (p <0.05).
The highest increase of eosinophil is in P3 treatment.
P0 was significantly different from P3, yet wasnot
significantly different from P1 and
P2.
At basophil count, the results were not
significantly different (p <0.05) when compared with
control (P0).
Leukocyte Count and Differential Leukocyte Count of Carp (Cyprinus carpio Linn) after Infected by Aeromonas salmonicida
547

The highest neutrophil increase is in P3 treatment.
P0 was significantly different from P1, P2, and P3,
whereas P1 was significantly different from P2 and
P3. P2 was significantly different from P0, P1, and
P3.
The highest lymphocyte increase is in P3
treatment. P0 differed significantly with P1, P2 and
P3, whereas P1 was not significantly different from
P2 but significantly different from P3. P2 was
significantly different from P0 and P3, yet was not
significantly different from P1.
The increase in monocytes was highest in
treatment P3. P0 was not significantly different from
P1 and P2; this was in contrast with P3.
Eosinophils are the second major cell of the
meiloid system. These cells are not as efficient as
neutrophils in phagocytosis, yet have lysosomes and
carry out a respiratory burst when precisely
stimulated (Tizard, 1982). Increased eosinophils as an
immune is a response to toxic and extracellular
enzymes produced by Aeromonas. Pathogenic
properties Aeromonas known as opportunistic
pathogens in humans and fish, involving some
extracellular enzymes, are reported to correlate with
the mechanisms of infection and invasion of these
bacteria (Rao et al., 1998).
Increased basophils occur due to the
inflammatory process (inflammation), leukemia, and
infective healing phases. Basophils are rarely found
in the blood circulation of fish. Decreased basophils
or basophenia may be caused by chemotherapy, in
pregnancy, hyperthyroidism, radiation, in acute
infection, and during treatment with glucocorticoids
(Bijanti et al., 2010). The glucocorticoid hormone is
one of the classes of the corticosteroid hormone. This
indicates that the decrease in basophil count affects
the production of corticosteroid hormones that play
one of them as a suppressor of the immune response.
Neutrophils are the first cells to respond to infection
by foreign bodies entering the fish body (Summers et al.,
2010). To respond to bacterial infection, neutrophils
leave the marginal group and enter the infection area and
the thymus release its source of reserve resulting in
increased granulopoiesis. The increase in granulopoiesis
can be seen because there are many immature
neutrophils that enter the blood circulation which is
called a shift to the left. As the main function of
neutrophils is phagocytosis (killing and digesting
microorganisms), acute bacterial infections and trauma
trigger neutrophil production. (Atmaja et al., 2016).
An increased number of lymphocytes can occur
due to stressful fish (Sakai, 1999). Stress can cause
non-specific immune response disorders, such as
lymphocyte proliferation (increase in cell count and
form changes into T cells and B cells). Lymphocytes
are cells that function to produce antibodies or as
effector cells in response to bound antigen
macrophages. The circulating lymphocytes primarily
originate from the thymus, some of which are
relatively immature differentiated, multiply cells, are
T lymphocytes. These, then then reenter the
bloodstream. T cells are responsible for cellular
immune reactions and have specific surface receptors
to recognize foreign antigens. Other lymphocytes
differentiate into B lymphocytes, by producing
humoral antibodies in the bloodstream and binding
specifically to foreign antigens causing phagocytosis,
cell lysis, and killer cells (killer cells or K cells) of
invading organisms. T cells and B cells
morphologically can only be distinguished when
activated by antigen (Tizard, 1982).
Increasing number of monocytes occurs because
bacteria are foreign agents that must be eliminated so
that monocytes will develop into macrophages to the
place of infection to perform the process of
phagocytosis. Inflammatory processes during tissue
damage by infection or antigen-antibody reactions will
increase monocyte production to two times more. The
circulation of monocytes in the blood becomes shorter.
Monocyte maturation which becomes macrophages
happens more quickly and immediately leads to
damaged tissue (Maftuch, 2007). The proportion of
monocytes is very low in the leukocyte population, but
may increase by about 38% in a short time if infection
occurs (Andayani et al., 2008).
4 CONCLUSIONS
Based on the results of , it can be concluded that
bacterial infections Aeromonas salmonicida in Carp
cause changes in leukocyte count and differential
leukocyte count. The change is an increase in the
number of leukocytes count while in the differential
leukocyte count, there is an increase in eosinophils,
neutrophils, lymphocytes and monocytes.
REFERENCES
Alamanda, I.E., N.S. Handajani, and A. Budiharjo. 2007.
Use of Hematology Method and Observation of
Blood Endoparasit for Determination of Dombo
Catfish Health (Clarias gariepinus) in Pond Culture
of Mangkubumen Village Boyolali. FMIPA
Sebelas Maret University. Surakarta. 34-38.
Andayani, R., Lisawati, Y., Maimunah. 2008.
Determination of Antioxidant Activity, Total
ICPS 2018 - 2nd International Conference Postgraduate School
548

Phenolic Levels, and Lycopene On Tomato Fruit.
Journal of Pharmaceutical Science and Technology.
13 (1).
Atmaja, A.S., K. Radius and D. Freddy. 2016. Laboratory
Examination to Distinguish Bacterial Infections
and Virus Infections. CDK-241. 43(6): 458.
Bijanti, R., A.Y.M. Gandul., S.W. Retno and R. Budi
Utomo. 2010. Veterinary Clinical Pathology
Books. Print I. Airlangga University Press.
Surabaya. 51-55.
Cahyono, B. 2000. Freshwater Fish Cultivation: Gouramy
Fish, Nile Fish, Gold Fish. Yogyakarta: Kanisius.
Irianto, A. 2005. Teleostei Fish Pathology. Gajah Mada
University. Yogyakarta. 256 .
Ministry of Maritime Affairs and Fisheries. 2014. Jakarta :
Ministry of Maritime Affairs and Fisheries.
Kumar, M.P. and K.S. Ramulu. 2013. Haematolgical
Changes In Pangasius Hypopthalamus Infected
With Aeromonas hydrophila. International Journal
of Food, Agriculture and Veterinary Sciences. 3
(1): 70-75.
Supriatna, Y. 2013. Fish Cultivation in Water-saving Pool.
Agromedia Library. Jakarta. 11-13.
Maftuch. 2007. Vibrio Alginolyticus Exposure to Fish
Hippopatology of Mice Grouper (Cromileptes
altivelis) and Increase in Amou and Macrophage
Cell Activity. Journal of Fisheries
Research Vol. 10 no.1. Faperik Unibraw. Hal. 66-
70.
Nitimulyo, K.H., I.Y.B. Lelono, and A. Sarono. 1993.
Description of Pests and Diseases of Classified Fish
Quarantine Bacteria Book 2. Agricultural
Quarantine Center. Jakarta.
Noercholis, A., M. Aziz, and M. Maftuch. 2013.Feature
Roundness Extraction for Counting the Number of
Leukocytes in Fish Blood Cell Imaging. Journal of
EECCIS. 7 (1).
Nuryati, S., N.A. Maswan, Alimuddin, Sukenda, K
Sumantadinata, F.H. Pasaribu, R.D. Soejoedono
and A. Santika. 2010. Blood Fish After Masion
Vaccinated with DNA Vaccine and Tested
Challenge with Koi Herpes Virus. Journal of
Aquaculture Indonesia. 9 (1): 9-15.
Rao MB et al. 1998. Molecular and Biotechnologi Aspect
of Microbial Proteases. Microbiol. Mol. Biol. Rev.
63 (3): 597-635.
Sakai, M. 1999. Current Research Status of Fish
Immunostimulants. J. Aquaculture. 172: 63-92.
Suhermanto, A., S. Andayani, and Maftuch. 2013.
Influence of Total Phenol Sea Cucumber
(Holothuria scabra) Against Non-Specific
Immune Response of Carp (Cyprinus carpio). 13
(2): 225-233.
Summers, R.W., D.E. Elliot, and J.V. Weinstock. 2010.
Trichuris suis might be effective in treating
allergic rhinitis. Journal of Allergy and Clinical
Imunology. 125: 766-767.
Tizard, I.R., 1982. An Introduction of Veterinary
Immunology. W. B. Saunders Company. 254-257.
Zainun, Z. 2007. Observation of the Hematologic
Parameters on Mas Fish Given
Immunostimulan. Center for Freshwater
Aquaculture Development. Sukabumi. 45-49.
Leukocyte Count and Differential Leukocyte Count of Carp (Cyprinus carpio Linn) after Infected by Aeromonas salmonicida
549
