
The Effect of Alpha-mangostin in Glucose Level, Cholesterol Level
and Diameter of the Islets of Langerhans of STZ-induced Diabetic
Mice
Saikhu Akhmad Husen
1,2
, Salamun
1
, Arif Nur Muhammad Ansori
3
, Raden Joko
Kuncoroningrat Susilo
1
, Suhailah Hayaza
1
and Dwi Winarni
1,2
1
Department of Biology, Faculty of Science and Technology, Universitas Airlangga, Surabaya, Indonesia
2
Animal Histology Laboratory, Faculty of Science and Technology, Universitas Airlangga, Surabaya, Indonesia
3
Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia
Keywords: Alpha-mangostin, antidiabetic, antilipidemic, diabetic mice, streptozotocin.
Abstract: The increasing prevalence of Diabetes Mellitus (DM) worldwide is an issue of major socio-economic
concern. DM is a complex and a multifarious group of disorders that disturbs the metabolism of
carbohydrates, fat, and protein. Medicinal plants play an important role in the management of DM,
especially in developing countries. The aim of this study is to investigate the antidiabetic and antilipidemic
effects of alpha-mangostin in STZ-induced diabetic mice. We conducted the study using the male BALB/C
mice. The mice were divided into two groups: the normal control (KN) and the STZ-induced diabetic mice.
Streptozotocin (STZ) induction was performed using multiple low-doses of 30 mg/kg bw injected for five
consecutive days. The diabetic mice were grouped again into three subgroups: diabetic control (KD),
metformin HCL treated diabetic mice (KM), and diabetic mice which were treated using alpha-mangostin at
2 mg/kg bw (P1), 4 mg/kg bw (P2), and 8 mg/kg bw (P3). Before and after STZ injection, the blood glucose
and the cholesterol levels were observed. The blood glucose and the cholesterol levels were also measured
on the 1
st
, 7
th
, and 14
th
day of alpha-mangostin treatments. Treatment was given for 14 days. At the 15
th
day,
the pancreases were collected and then processed into histological slides. The results of this experimental
study indicated that alpha-mangostin has hypoglycemic and hypolipidemic activities which can ameliorate
the damaged islets of Langerhans in STZ-induced diabetic mice. Therefore, we concluded that alpha-
mangostin is a promising antidiabetic and antilipidemic agent due to its antioxidant activity.
1 INTRODUCTION
DM is a metabolic disorder that affects about 6% of
the world population. DM is characterized by the
prolonged hyperglycemic conditions due to the
reduction of both insulin secretion and insulin
sensitivity (Kang et al., 2014). DM is divided into
type-1 DM (insulin dependent DM) and type-2 DM
(non-insulin dependent DM). Type-1 DM is an
autoimmune disease which causes the immune
system to attack pancreatic cells, thus damaging a
person's ability to produce insulin. Type-2 DM is a
metabolic disorder that is characterized by an insulin
resistance, a decrease of cell sensitivity to insulin,
and a relative lack of insulin due to the damage
suffered by pancreatic islet β-cells (American
Diabetes Association, 2013). The decrease of cell
sensitivity to insulin is a typical condition, as well as
the cause of type-2 DM. Progressive decrease in
insulin secretion is generally a result of decreased
tissue sensitivity to insulin (McClung et al., 2004;
Husen et al., 2017a; Husen et al. 2017b).
Beside the prolonged hyperglycemic conditions,
one of the factors which causes DM is obesity due to
the increased levels of fat in the body caused by
hyperlipidemic and escalating levels of cholesterol
in the blood (Husen et al., 2016; Husen et al.,
2017a). The increased blood cholesterol levels can
be followed by the dilated levels of free fatty acids,
which generate the enlarged superoxide production
by mitochondria and the higher risk of cell exposure
by the reactive oxygen species (ROS). A growth in
superoxide production will lead to an increase in
nitric oxide (NO) caused by the induction of NO
synthase enzymes. This condition leads to the
Husen, S., Salamun, ., Ansor i, A., Susilo, R., Hayaza, S. and Winarni, D.
The Effect of Alpha-mangostin in Glucose Level, Cholesterol Level, and Diameter of the Islets of Langerhans of STZ-induced Diabetic Mice.
DOI: 10.5220/0007547005610566
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 561-566
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
561

production of reactive nitrogen species (RNS),
which will oxidize the sulfhydryl groups of proteins,
especially amino nitrate acids such as tyrosine,
which can increase lipid peroxidation and cause a
harmful DNA damage to cells (Novelli et al., 2010;
Husen et al., 2017a).
The condition of hyperlipidemia in the people
with obesity can boost the oxidative stress in the
body, which can lead to some complications. People
with obesity also experience heightened levels of
cholesterol in the body (hypercholesterolemia)
caused by excessive accumulation of fat in the body.
One of the negative effects of obesity is the insulin
resistance, which is the inability of insulin to
produce the normal biological functions, which
causes decreased tissue sensitivity to insulin. The
cell resistance to cellular action of insulin is
developed in people with obesity, which is
characterized by the reduced ability of insulin to
support the glucose intake in fat and muscle
resulting in the hyperglycemic conditions (Husen et
al., 2017a; Husen et al., 2017b). The condition of
hyperglycemia leads directly to the increased levels
of ROS and RNS. ROS and RNS can directly
oxidize and destroy DNA, proteins, and lipids. High
levels of ROS and RNS also can damage the
macromolecules indirectly, which causes oxidative
stress. Oxidative stress occurs when there is an
imbalance between the number of highly reactive
molecules (ROS and RNS) with the existing
antioxidants (Novelli et al., 2010; Husen et al., 2018;
Ansori et al., 2018).
Antioxidants are substances that can prevent the
negative effects of free radicals by providing
electrons to enable to suppress damages of lipids,
cell membranes, blood vessels, DNA, and other
damages caused by the reactive compounds, such as
ROS and RNS. To reduce the occurrence of the free
radical’s effect, extra antioxidants from the outside
(exogenous), such as vitamin E, vitamin C and other
antioxidants obtained from consuming various types
of fruits and vegetables that contain high
antioxidants, are needed. One type of antioxidants
which still provides a chance to overcome free
radicals up until today is alpha-mangostin. The
alpha-mangostin compound is a pigment of Garcinia
mangostana, which is able to contribute hydrogen
atoms and stabilize the free radicals by resonance,
which is hard to participate in other radical reactions
(Husen et al., 2018). In addition to neutralizing free
radicals, antioxidants are expected to reduce
oxidative stress, mainly in various cells affected by
the prolonged hyperglycemic conditions, such as
hepatocyte cells in the liver and renal tubular cells
(Vallon, 2011; Ansori et al., 2018).
Indonesia has a high number of biodiversity,
which contains various natural potentials that can be
utilized for the treatment of various diseases
(Wahyuni et al., 2017; Ansori et al., 2018). One of
Indonesia's original flora which currently has great
potential to be developed as a medicinal raw
material is mangosteen (Ansori et al., 2018). The
pericarp of the mangosteen fruit contains an active
compound known as xanthone. Beside having anti-
hypertensive and anti-inflammatory potential,
xanthone compounds also play an important role as
a powerful antioxidant compared to vitamin C and
vitamin E in preventing free radicals and cell
damage, as well as inhibiting cell degeneration
processes (Chin et al., 2008). The xanthone
compounds contained in the pericarp of the
mangosteen, especially alpha-mangostin
compounds, have been proven ameliorate the
damaged pancreatic islet β-cells so that insulin can
be produced optimally (Husen et al., 2017b; Husen
et al., 2018). Based on the above background
information, it has now been widely reported that the
raw mangosteen pericarp extract is able to lower
glucose and blood cholesterol levels. However,
presently there has been no scientific explanation
about the potential of alpha-mangostin to reduce
blood glucose and cholesterol levels, as well as to
ameliorate the pancreatic islet β-cells damages
caused by the prolonged hyperglycemic conditions.
Thus, this study was aimed to investigate the effects
of alpha-mangostin in glucose level, cholesterol
level, and diameter of the islets of Langerhans of
STZ-induced diabetic mice.
2 MATERIALS AND METHODS
This study was conducted at the Animal Laboratory,
Animal Histology Laboratory, and Molecular
Genetics Laboratory, Faculty of Science and
Technology, Universitas Airlangga. The ethical
clearance for this study was obtained from the
Committee of Animal Care and Use, Faculty of
Veterinary Medicine, Universitas Airlangga (701-
KE). The used samples were adult male mice of
BALB/C strain aged 3-4 months with weights
ranging from 30 to 40 g. The study materials consist
of alpha-mangostin and STZ (purchased from
Sigma), buffer citrate solution pH 4.5, phosphate-
buffered saline (PBS), solvent extract of
carboxymethylcellulose (CMC), standard
antidiabetic drugs (Metformin HCl 100 mg/kg bw),
ICPS 2018 - 2nd International Conference Postgraduate School
562
lard, xylazine and ketamine, and glucose (10% D-
glucose in aquadest). The main tools used were mice
cage made in plastic with lid of gauze wire, drinking
bottle, feeding plate, husk, microscope, Petri dish,
analytical scale, injection needles which have lead
tackle at the end, 1 mL insulin injection needle for
diabetic induction, Accu-Check® Active Test,
EasyTouch® GCU Multi-Function Monitoring
System, glass tools, and rotary vacuum evaporator
(Buchi).
The study samples consisted of 24 male mice,
distributed to the normal control group (KN) and
diabetic group (induced by STZ). The fasting blood
glucose and fasting blood cholesterol were measured
before and after the STZ induction. The
measurement of fasting blood glucose was
performed on the 7
th
and 14
th
day after induction of
STZ. Meanwhile, the measurement of blood glucose
levels was performed using a glucometer to
determine the diabetic condition of mice. Only the
mice which had the fasting blood sugar level of
more than 140 mg/dL were used as a diabetic mice
group. The grouping of experimental animals was
performed as follows: non-diabetic mice were used
as the normal control group (KN), the diabetic mice
induced by STZ were divided into 2 control groups,
namely the diabetic control group (KD), the diabetic
control group which was given Metformin HCl of
dose 100 mg/kg bw (KM), and, the last one, was the
alpha-mangostin treatment group. Furthermore, the
alpha-mangostin treatment group was divided into 3
subgroups, which were P1 which was given 2 mg/kg
bw, P2 which was given 4 mg/kg bw, and P3 which
was given 8 mg/kg bw. All treatments were
administered for 14 days.
The measurements of fasting blood glucose and
fasting blood cholesterol levels were performed in
all groups of mice before and after STZ induction,
the measurement of which then continued on the 1
st
,
7
th
, and the 14
th
day of the alpha-mangostin
treatment. The measurement of fasting blood
glucose was done by using Accu-Check® Active
Test, while the measurement of cholesterol levels
was performed using EasyTouch® GCU Multi-
Function Monitoring System. The measurements of
fasting blood glucose and cholesterol levels were
performed after the mice were fasted for 6-8 hours.
3 RESULTS AND DISCUSSION
The mean of mice’s fasting blood glucose level and
fasting blood cholesterol level data, before and after
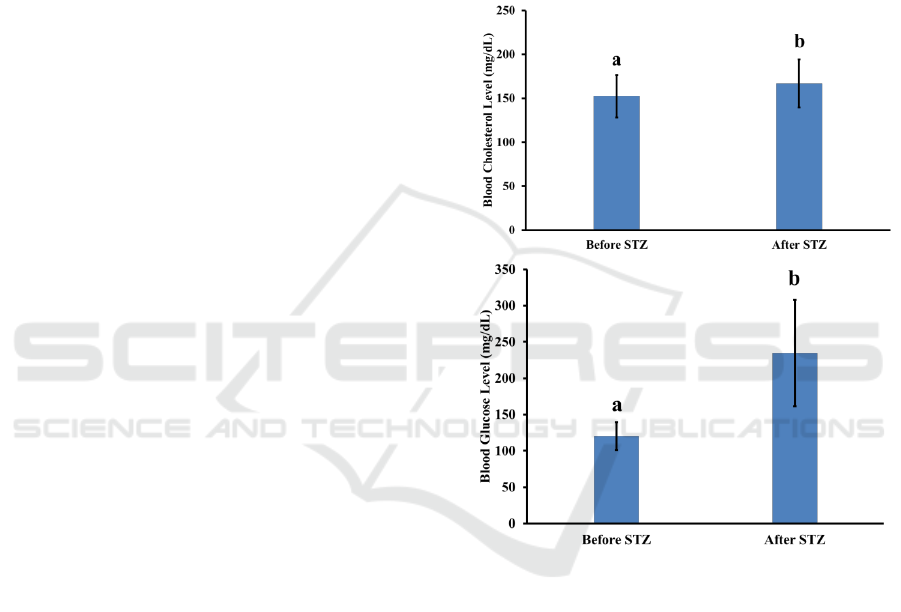
STZ induction, are presented in Figure 1. The mean
of mice’s fasting blood glucose and fasting blood
cholesterol level data after alpha-mangoostin
treatment is presented in Figure 2, while the mean
data of diameter of the islets of Langerhans after the
administration of alpha-mangostin is shown in
Figure 3.
Figure 1: Blood glucose and cholesterol level (mg/dL)
before and after STZ induction. The different letter
indicated a significant difference.
The Effect of Alpha-mangostin in Glucose Level, Cholesterol Level, and Diameter of the Islets of Langerhans of STZ-induced Diabetic Mice
563
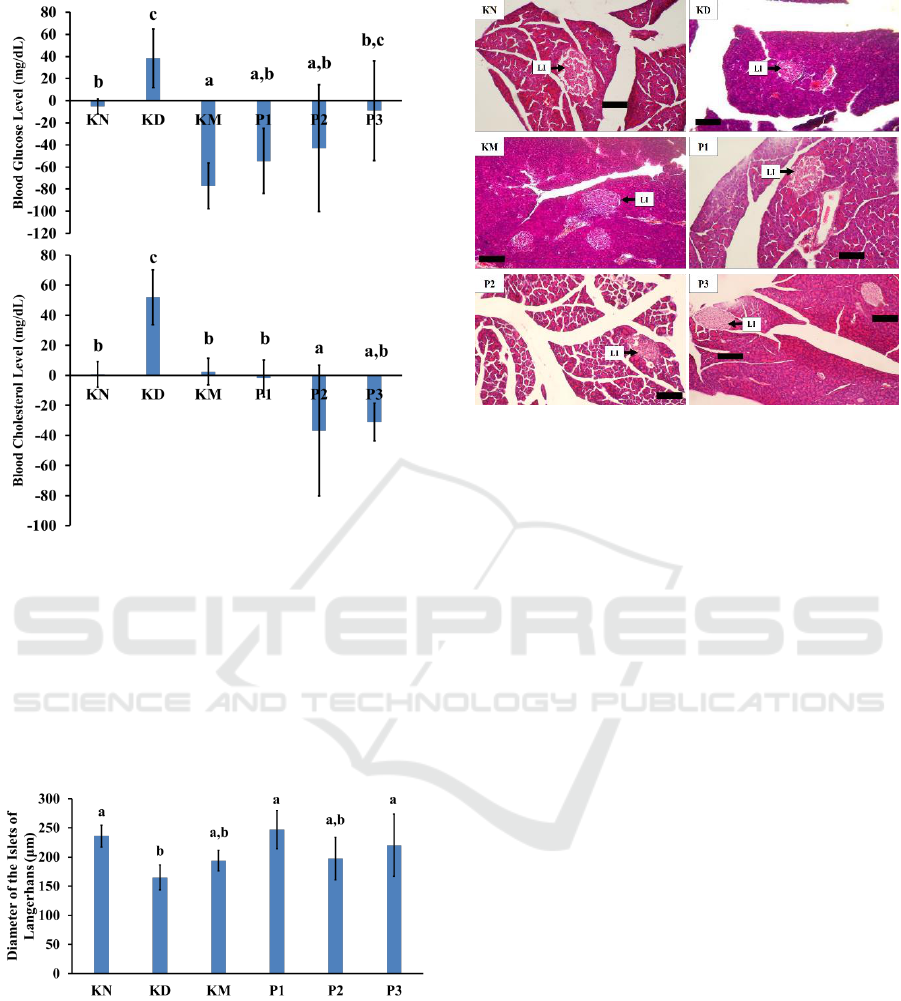
Figure 2: Blood glucose level and cholesterol level
changes of each mice groups after treatments. The same
letters above diagrams indicated an insignificant
difference, while different letter indicated a significant
difference. KN: normal control group; KD: diabetic group
without metformin HCl; KM: diabetic group with
metformin HCl 100 mg/kg bw; P1: diabetic treatment
group with alpha-mangostin 2 mg/kg bw; P2: diabetic
treatment group with alpha-mangostin 4 mg/kg bw; P3:
diabetic treatment group with alpha-mangostin 8 mg/kg
bw.
Figure 3: Diameter of the islets of Langerhans of each
mice group after treatments. Same letters above the
diagrams indicate insignificant difference, while different
letters indicate significant difference. KN: normal control
group; KD: diabetic group without metformin HCl; KM:
diabetic group with metformin HCl 100 mg/kg bw; P1:
diabetic treatment group with alpha-mangostin 2 mg/kg
bw; P2: diabetic treatment group with alpha-mangostin 4
mg/kg bw; P3: diabetic treatment group with alpha-
mangostin 8 mg/kg bw.
Figure 4: Histological structure of pancreatic gland and
diameter of the islets of Langerhans of each mice group
after treatments. KN: normal control group; KD: diabetic
group without metformin HCl; KM: diabetic group with
metformin HCl 100 mg/kg bw; P1: diabetic treatment
group with alpha-mangostin 2 mg/kg bw; P2: diabetic
treatment group with alpha-mangostin 4 mg/kg bw; P3:
diabetic treatment group with alpha-mangostin 8 mg/kg
bw; LI: diameter of the islet of Langerhans; Bar: 100 µm.
Lard was administered for 3 weeks to obtain
hyperlipidemic condition within the mice.
Hyperlipidemic state has a great chance to cause
insulin resistance, so it is very easy to occur after
administration of STZ. This study began with the
administration of lard followed by STZ. The
measurements of fasting blood glucose level and
fasting blood cholesterol level (mg/dL) were done
before and after the STZ injection. The data is
presented in Figure 1, it shows that STZ injection
with a dose of 30 mg/kg body weight for five
constitutive days was able to significantly increase
the mice’s blood glucose levels significantly, from a
mean blood glucose level of 120.625±19.354 to
183.500±39.419. STZ is a nitric oxide (NO) donor
and NO was found to bring about the destruction of
pancreatic islet cells and contribute to DNA damage
in the cells (Kröncke et al., 1995). This condition
indicates that STZ as an oxidant agent is capable of
destroying pancreatic islet β-cells, which leads to the
decrease of insulin production. Therefore, it
generates an increased level of fasting blood glucose
(Husen et al., 2016). Meanwhile, on the blood
cholesterol levels, the STZ injection was also able to
increase the mice’s blood cholesterol level
significantly from a mean of 152.40±24.294 before
injection to 167.000±27.325 after injection. This
ICPS 2018 - 2nd International Conference Postgraduate School
564

means that STZ is a highly reactive free radical
which is able to increase ROS and RNS levels in
cells, especially for insulin-sensitive cells and tissues
such as the pancreatic gland. In the current work,
diabetes was induced in laboratory mice via
intraperitoneal injection of STZ. Streptozotocin
(STZ) is considered to be toxic to insulin producing
beta cells within pancreas, and thus it is widely used
to induce experimental diabetes in laboratory
animals (Aldahmash et al., 2015). Ansori et al.
(2018) showed pathological changes in kidney of
STZ-induced diabetic mice.
The increased levels of blood cholesterol after
STZ injection caused by the prolonged
hyperglycemic conditions were the result of the
cellular damage of pancreatic islet β-cells and the
decrease in insulin levels in blood. These conditions
led to the increase of gluconeogenesis and lipolysis
in striated muscle and fat tissues, as well as fat
mobilization of adipose tissue, which caused the
increased level of cholesterol in blood. The
breakdown of fatty tissue both within the striated
muscle cells and within the tissues of the body can
lead to the increased levels of cholesterol in the
blood (Husen et al., 2016; Husen et al., 2017a). In
the prolonged hyperglycemic conditions, the
administration of exogenous antioxidant compounds
such as alpha-mangostin was expected to provide
hope for ameliorating of the pancreatic islet β-cells
damaged by free radicals, such as ROS and RNS.
Based on this study, it was found that the
administration of alpha-mangostin antioxidants
affects average fasting blood glucose levels in the
diabetic mice. The dose of 2 mg/kg body weight had
the highest response compared to the other treatment
groups with doses of 4 and 8 mg/kg body weight.
Those results showed a condition in which the
lowest dose of the alpha-mangostin antioxidant
active substance was able to provide a more positive
response, compared to the larger dose groups. It has
been proven that the antioxidant compounds of
alpha-mangostin is a powerful antioxidant and has
the ability to restore the homeostatic condition of
glucose levels in the blood, called hormesis.
Hormesis is a term in toxicology that demonstrates
the phenomenon of response to the low doses
stimulation and inhibition at the high doses and
results in a curve formed of inverted J or U (Husen
et al., 2017b).
The glucose cannot be processed into energy
because of the hyperglycemic condition. Therefore,
the energy must be made from other sources, such as
fat and protein. Energy is obtained through the
increased catabolism of protein and fat. Along with
these conditions, there is a stimulation of lipolysis
and the increase levels of free fatty acids and blood
glycerol. This leads to the increasing production of
acetyl-CoA by the liver, which in turn is converted
to acetoacetic acid and ultimately reduced into β-
hydroxybutyric acid or decarboxylated into acetone
(Husen et al., 2016; Husen et al., 2017a). Due to the
formation of energy from proteins and fats, the
cholesterol levels formed in the chain of fat and
protein metabolism increase. In patients with DM,
hyperglycemic conditions lead to the increased
production of ROS and RNS due to the increase of
NADPH oxidation in endothelial tissue. ROS and
RNS are highly reactive molecules that can directly
oxidize and destroy DNA, proteins, and lipids and
can cause an oxidative stress. An oxidative stress
occurs when there is an imbalance between the
number of highly reactive molecules (ROS and
RNS) with the existing antioxidants (Husen et al.,
2018; Ansori et al., 2018).
Interestingly, this study has proved that most
diabetic mice have high cholesterol levels, as shown
in the KD group, due to the interference of fat
metabolism which causes high levels of acetate as
one of the cholesterols formed in one reaction
catabolism. Excessive energy sources lead to an
excessive acetate formation, and fat in the body will
increase as well. The increased fat metabolism
causes the occurrence of abnormal fat metabolism
with cholesterol deposits in the blood vessel wall.
This condition can lead to an atherosclerosis and a
decrease protein in the body. Various diseases are
often associated with the increased cardiovascular
risk parameters such as hypertriglyceridemia,
hypercholesterolemia, and high-density lipoprotein
(HDL) (Höhn et al., 2014).
4 CONCLUSIONS
We found that the administration of STZ can
increase the fasting blood glucose levels and the
fasting blood cholesterol levels in STZ-induced
diabetic mice significantly. In addition, the
administration of alpha-mangostin can reduce the
average of fasting blood glucose level and fasting
blood cholesterol level, as well as ameliorate the
pancreatic islet β-cells damaged by STZ
administration. Therefore, we concluded that alpha-
mangostin is a promising antidiabetic and
antilipidemic agent due to its antioxidant activity.
The Effect of Alpha-mangostin in Glucose Level, Cholesterol Level, and Diameter of the Islets of Langerhans of STZ-induced Diabetic Mice
565

ACKNOWLEDGEMENTS
The authors would like to thank the Dean of Faculty
of Science and Technology and Head of Institute of
Innovation and Research Universitas Airlangga for
the opportunity given to conduct this research,
which is funded by a grant from Directorate General
of Higher Education, Ministry of Research,
Technology, and Higher Education of the Republic
of Indonesia awarded to Saikhu Akhmad Husen
(Associate Professor in Faculty of Science and
Technology, Universitas Airlangga). Moreover, the
authors would liketo especially thank the PMDSU
Scholarship - Batch III (Ministry of Research,
Technology, and Higher Education of the Republic
of Indonesia) awarded to Arif Nur Muhammad
Ansori, Raden Joko Kuncoroningrat Susilo, and
Suhailah Hayaza.
REFERENCES
American Diabetes Association. 2013. Standards of
medical care in diabetes—2013. Diabetes Care. 36
(Suppl. 1): S11-S66.
Aldahmash B.A., El-Nagar, D.M., Ibrahim, K.E., and
Metwaly, M.S. 2015. Biotin amelioration of
nephrotoxicity in streptozotocin-induced diabetic
mice. Saudi Journal of Biological Sciences. 22(5):
564-9.
Ansori, A.N.M., Susilo, R.J.K., Hayaza, S., Winarni, D.,
and Husen, S.A. 2018. Renoprotection by Garcinia
mangostana L. pericarp extract in streptozotocin-
induced diabetic mice. Iraqi Journal of Veterinary
Sciences, article in press.
Chin, Y.W., Jung, H.A., Chai, H., Keller, W.J., and
Kinghorn, A.D. 2008. Xanthones with quinone
reductase-inducing activity from the fruits of Garcinia
mangostana (Mangosteen). Phytochemistry. 69: 754-
758.
Höhn, A., Jung, T., and Grune, T. 2014.
Pathophysiological importance of aggregated damaged
proteins. Free Radical Biology and Medicine. 71: 70-
89.
Husen, S.A., Kalqutny, S.H., Ansori, A.N.M., Susilo,
R.J.K., Alymahdy, A.D., and Winarni, D. 2017b.
Antioxidant and antidiabetic activity of Garcinia
mangostana L. pericarp extract in streptozotocin-
induced diabetic mice. Bioscience Research. 14(4):
1238-1245.
Husen, S.A., Khaleyla, F., Ansori, A.N.M., Susilo, R.J.K.,
and Winarni, D. 2018. Antioxidant activity assay of
alpha-mangostin for amelioration of kidney structure
and function in diabetic mice. Advances in Social
Science, Education, and Humanities Research. 88:84-
88.
Husen, S.A., Winarni, D., Khaleyla, F., and Kalqutny,
S.H. 2016. Activity test of various mangosteen
(Garcinia mangostana) pericarp extract fractions to
decrease fasting blood cholesterol levels and lipid
peroxidation activity in diabetic mice. Journal of
Biological Researches. 22(1): 13-17.
Husen, S.A., Winarni, D., Khaleyla, F., Kalqutny, S.H.,
and Ansori, A.N.M. 2017a. Activity assay of
mangosteen (Garcinia mangostana L.) pericarp
extract for decreasing fasting blood cholesterol level
and lipid peroxidation in type-2 diabetic mice. AIP
Conference Proceedings. 1888.
Kang, K.S., Lee, W., Jung, Y., Lee, J.H., Lee, S., Eom, D.,
Jeon, Y., Yoo, H.H., Jin, M.J., Song, K.I., Kim, W.J.,
Ham, J., Kim, H.J., and Kim, S. 2014. Protective
effect of esculin on streptozotocin-induced diabetic
renal damage in mice. J. Agric. Food Chem. 62: 2069-
2076.
Kröncke, K.D., Fehsel, K., Sommer, A., Rodriguez, M.L.,
and Kolb-Bachofen, V. 1995. Nitric oxide generation
during cellular metabolization of the diabetogenic N-
methyl-N-nitroso-urea streptozotozin contributes to
islet cell DNA damage. Biol Chem Hoppe Seyler.
376(3): 179-185.
McClung, J.P., Roneker, C.A., Mu, W., Lisk, D.J.,
Langlais, P., Liu, F., and Lei, X.G. 2004.
Development of insulin resistance and obesity in mice
overexpressing cellular glutathione peroxidase. PNAS.
101(24): 8852-8857.
Novelli, M., Bonamassa, B., Masini, M., Funel, N.,
Canistro, D., De Tata, V., Martano, M., Soleti, A.,
Campani, D., Paolini, M., and Masiello, P. 2010.
Persistent correction of hyperglycemia in
streptozotocin-nicotinamide-induced diabetic mice by
a non-conventional radical scavenger. Naunyn
Schmiedebergs Arch Pharmacol. 382(2): 127-137.
Vallon, V. 2011. The proximal tubule in the
pathophysiology of the diabetic kidney. American
Journal of Physiology-Regulatory, Integrative and
Comparative Physiology. 300: R1009-R1022.
Wahyuni, D.K., Ansori, A.N.M., and Vidiyanti, F. 2017.
GC-MS analysis of phytocomponents in methanolic
extracts of leaf-derived callus of Justicia gendarussa
Burm.f. Bioscience Research. 14(3): 668-677.
ICPS 2018 - 2nd International Conference Postgraduate School
566
