
Preparation and Optimization of Natural Composite Oil
Absorbing Materials
Q S Wang
1
, L N Zheng
1, 2,*
, H N Chen
1
, Y W Pan
1
, H Jiang
1
and G Tian
1
1
College of Marine Technology and Environmental, Dalian Ocean University,
Dalian 116023, China
2
Key Laboratory of Nearshore Marine Environmental Research, Dalian 116023,
China
Corresponding author and e-mail: L N Zheng, 8601375@qq.com
Abstract. In this paper, by using the graft copolymerization method, butyl methacrylate and
styrene were used as grafting monomers, benzoyl peroxide as initiator, and methylene
bisacrylamide as cross-linking agent, respectively, for natural waste bagasse. The powder
(RMC:Natural waste bagasse powder) was chemically modified to determine the grafting
monomer, initiator, amount of cross-linking agent, and the most suitable reaction time,
temperature and other conditions through orthogonal tests, thereby obtaining two kinds of
natural composite efficient oil absorption materials BMC(Natural oil absorption material) .
1. Introduction
While China’s economy is pursuing high-speed GDP growth, it has also brought with it a series of
ecological and environmental pollution issues.The problem of marine pollution is particularly
acute.The cellulose in bagasse is a very important renewable resource. Therefore, it is of practical
significance to carry out energy production of bagasse through an energy-saving and efficient
method.The search for an economical, efficient, and environmentally friendly technology to remove
oil pollutants is an urgent task facing the current treatment of offshore oil pollution.At present, there
are many methods to deal with oil pollution.This paper regards adsorb oil.[1]Chemical modification
of dried and ground bagasse powder by graft copolymerization.The modification conditions were
optimized to determine the optimal time, temperature, graft monomer content and crosslinker and
other modification conditions, so as to study the preparation of highly efficient oil adsorption
materials.
2. The modified experiment
2.1. Experimental reagents and equipment
The reagents and instruments used in the experiments are shown in Table 1 and Table 2:
Wang, Q., Zheng, L., Chen, H., Pan, Y., Jiang, H. and Tian, G.
Preparation and Optimization of Natural Composite Oil Absorbing Materials.
In Proceedings of the International Workshop on Environmental Management, Science and Engineering (IWEMSE 2018), pages 83-88
ISBN: 978-989-758-344-5
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
83

Table 1. Reagents of experiment.
Reagent
Technical level
Factory
Butyl methacrylate
Analytical purity
Tianjin Damao Chemical Reagent
Factory
Methylenebisacrylamide
Analytical purity
Tianjin Aolan Fine Chemical
Research Institute
Benzoyl peroxide
Analytical purity
Tianjin Fuchen Chemical Reagent
Factory
Toluene
Analytical purity
Sinopharm Group Chemical Reagent
Co., Ltd.
Nitrogen
99.9%High purity
Dalian High Purity Nitrogen Plant
Acetone
Analytical purity
Tianjin Damao Chemical Reagent
Factory
Anhydrous ethanol
Analytical purity
Sinopharm Group Chemical Reagent
Co., Ltd.
Pure water
purity
Dalian Ocean University Chemical
Analysis Laboratory
Table 2. Apparatus of experiment.
Instrument
Production model
Factory
Collector type magnetic stirrer
DF-101S
Jiangsu Jintan Zhengji Instrument
Co., Ltd.
Analytical electronic balance
METTLER TOLEDO Instrument
Co., Ltd.
Electric blast drying box
101
Shanghai Experimental Instrument
Factory
Diaphragm vacuum pump
GM-0.33Ⅱ
Tianjin Jinteng Experimental
Equipment Co., Ltd.
Chinese medicine machinery
grinder
RH-800
Zhejiang Ronghao Industry and
Trade Co., Ltd.
Soxhlet extractor
Shenyang Chemical Instrument
Factory
2.2. Experimental method
The waste bagasse after the juice was washed with tap water and pure water in this order, dried in an
oven, and pulverized into a powder with a pulverizer.The desired material was screened using a 40
mesh (particle size approx. 0.425 mm) sieve and placed in a desiccator for use.
Weigh 3.00g of spare raw material bagasse (particle size is about 0.5mm) into a 500mL three-
necked flask with an electronic balance, and pour 300mL of pure water into a three-necked bottle
containing bagasse powder and put it into a thermostatic magnetic stirrer.Three bottles of high-purity
nitrogen were introduced into the bottle for 10 minutes to drive off the air in the bottle.[2]The amide,
as well as the quantitative grafting monomer, butyl methacrylate, react for a certain period of time. In
the experiment, the entire set of experimental devices was placed in a fume hood.During the reaction
process, the constant nitrogen flow was always maintained.The purpose was to avoid the presence of
air, which interrupted the polymerization reaction and affected the smooth progress of the reaction.
After the reaction was completed, the nitrogen valve and the thermostatic water bath stirrer power
were turned off, the three-necked flask was taken out, and the membrane vacuum pump was used to
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
84
remove heat, while washing with ethanol and pure water several times.[3]The filtered modified
material was placed in a glass petri dish and placed in a 60°C oven for 24 hours to dry.The dried
material was Soxhlet extracted with toluene for 12 hours in order to remove the homopolymer
formed by the reaction and obtain a purified modified material.After washing several times with
ethanol and pure water, it was dried in an oven at 60°C for 24 hours to obtain a purified modified
material.
3. Results and discussion
3.1. Effect of reaction temperature on chemical modification of materials.
In this group of experiments, the reaction time was set to 3 h, 4 ml of butyl methacrylate graft
monomer was added, 0.2 g of initiator benzoyl peroxide, and 0.02 g of crosslinker methylene
bisacrylamide.Then the graft copolymerization reaction was carried out at the reaction temperature of
45°C, 55°C, 65°C, 75°C, 85°C, and 95°C, and the oil absorption of the modified material in pure
diesel fuel was measured at different reaction temperatures in order the amount.
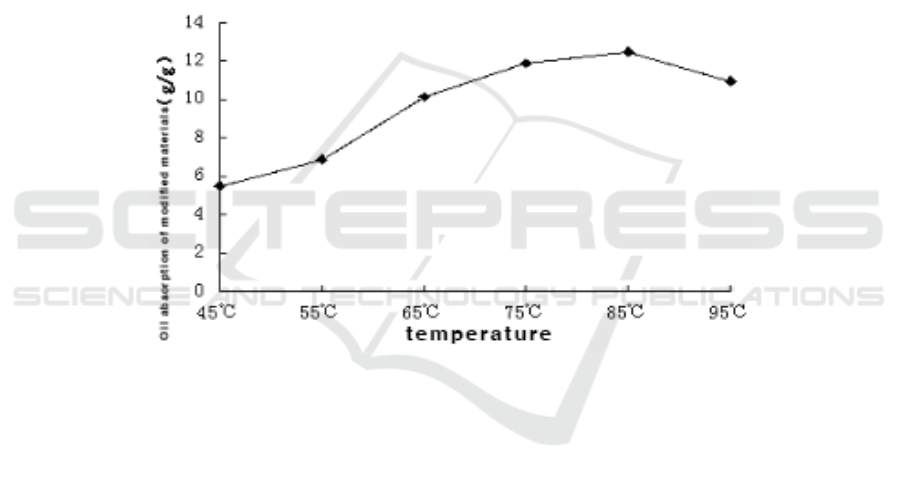
Figure 1. Effect of temperature on BMC.
It can be seen from Figure 1 that the oil absorption of the modified material BMC changes with
the temperature of the graft copolymerization reaction.When the reaction temperature is 45°C, the oil
absorption of the modified material is 5.50 g/g.With the gradual increase of the temperature, the oil
absorption of the modified material gradually increases.When the temperature is 85°C, the oil
absorption of the modified material reaches a maximum of 12.48 g/g.When the reaction temperature
continues to increase, the oil absorption of the modified material not only does not increase, but
gradually decreases.From this, it can be seen that the reaction temperature increases and the graft
copolymerization reaction rate increases, but when the reaction temperature is too high, the reaction
rate decreases, so that the oil absorption rate of the prepared modified material decreases.
3.2. Effect of reaction time on chemical modification of materials
In this group of experiments, the reaction temperature was set to 85° C, 4 mL of butyl methacrylate
grafting monomer, 0.2 g/g of initiator benzoyl peroxide, and 0.02 g of crosslinker methylene
bisacrylamide were added.[4]Then at the reaction temperature of 1h, 2h, 3h, 4h, 5h, 6h, the graft
copolymerization reaction was carried out and the oil absorption of the modified material pure diesel
oil under different conditions was determined in order.
Preparation and Optimization of Natural Composite Oil Absorbing Materials
85
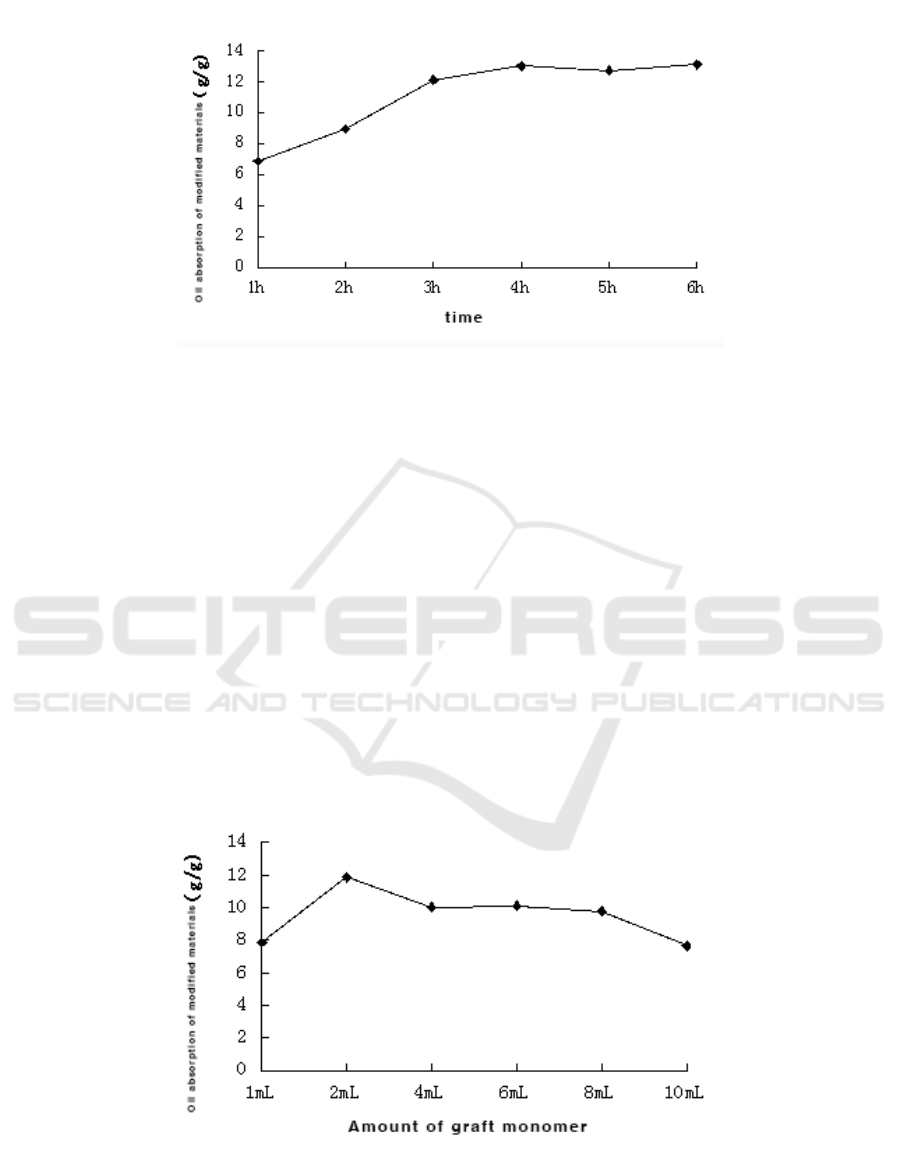
Figure 2. Effect of time on BMC.
As can be seen from Figure 2, the oil absorption of the modified material changes with the graft
copolymerization reaction time.When the reaction time is 1 h, the modified material has an oil
absorption of 6.85 g/g.With the gradual increase of time, the oil absorption of the modified material
also gradually increased.When the time was 4 hours, the oil absorption of the modified material
reached a maximum of 13.02 g/g.When the reaction time continues to increase, the oil absorption of
the modified material remains basically unchanged, indicating that the graft copolymerization
reaction has reached its maximum equilibrium.
3.3. Effects of grafting monomers on chemical modification of materials
In this group of experiments, the reaction temperature was set at 85° C, the reaction time was 3 hours,
0.2 g of benzoyl peroxide was added as initiator, and 0.02 g of methylene bisacrylamide was used as
the crosslinking agent.[5]Then the graft copolymerization reaction was performed under the
conditions of 1 ml, 2 ml, 4 ml, 6 ml, 8 ml, and 10 ml of graft monomer, and the oil absorption of the
modified material in the pure diesel oil was determined sequentially under the grafting monomer
condition.
Figure 3. Effect of grafting monomer’s amount on BMC.
As can be seen from Figure 3, when the grafting monomer butyl methacrylate was added in an
amount of 1 mL, the oil absorption of the modified material was 7.85 g/g.When the amount of
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
86
grafting monomer is 2 mL, the maximum balance of the oil absorption of the modified material is
11.85 g/g.When the amount of grafted monomer continues to increase, the oil absorption of the
modified material BMC begins to gradually decrease.
3.4. Effect of initiators on chemical modification of materials
In this group of experiments, the reaction temperature was set to 85° C, the reaction time was 3 hours,
and 4 mL of the grafting monomer butyl methacrylate and 0.02 g of the cross-linking agent
methylene bisacrylamide were added.Then, the reaction was carried out under the conditions of 0.1 g,
0.2 g, 0.3 g, 0.4 g, and 0.5 g of initiator benzoyl peroxide, and the oil absorption of different modified
materials in pure diesel oil was measured sequentially.
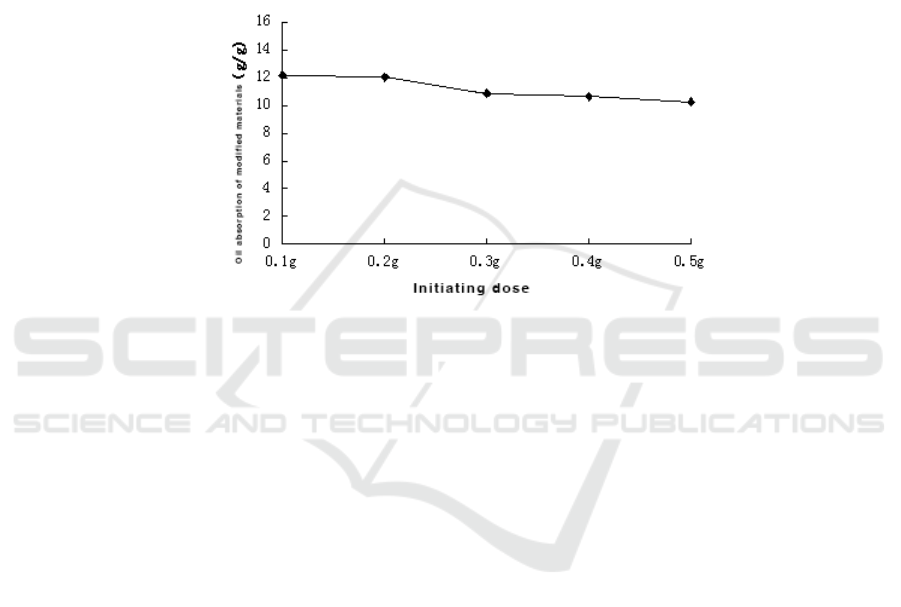
Figure 4. Effect of initiator’s amount on BMC.
As can be seen from Figure 4, when the amount of initiator benzoyl peroxide is 0.1g, the oil
absorption of the modified material is 12.20g/g, with the increase of the dose, the modified material
BMC The oil absorption rate gradually decreases.The reason is that an appropriate amount of
initiator can accelerate the reaction rate.However, when the amount of initiator is increased, the
amount of primary radicals generated per unit time will be increased, thereby increasing the
probability of termination reaction of the activity of the fiber branches, resulting in grafting. The rate
of copolymerization declines.Since benzoyl peroxide is in the form of small crystal particles, the
amount of 0.1 g when weighed is already very small, so no study has been conducted on the effect of
initiating a dose of less than 0.1 g.
3.5. Effect of crosslinking agents on chemical modification of materials
In this group of experiments, the reaction temperature was set to 85° C, the reaction time was 3 hours,
and 4 mL of the grafting monomer butyl methacrylate and 0.2 g of the initiator benzoyl peroxide
were added.Then, under the conditions of the amount of crosslinker methylenebisacrylamide dosing
0.01g, 0.02g, 0.03g, 0.04g, 0.05g, the graft copolymerization reaction was carried out and measured
sequentially under different conditions. Oil absorption in pure diesel.
Preparation and Optimization of Natural Composite Oil Absorbing Materials
87
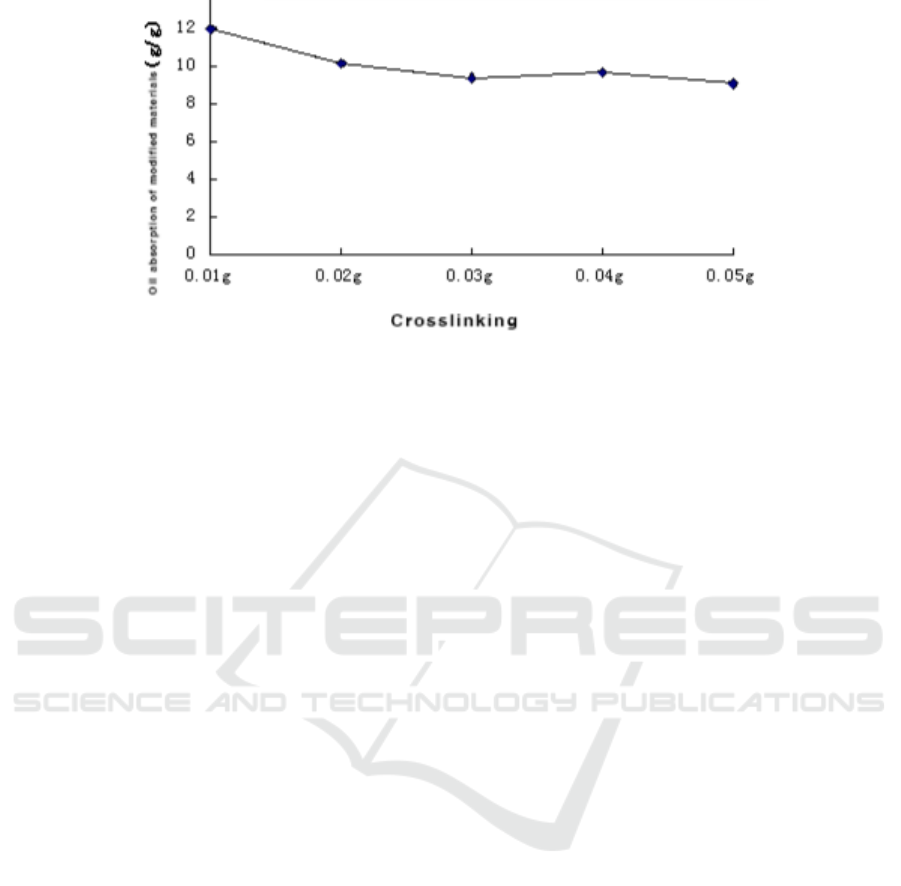
Figure 5. Effect of crosslinking agent’s amount on BMC.
As can be seen from Figure 5, when the crosslinking agent methylenebisacrylamide dosing
amount is 0.01g, the modified material has an oil absorption of 11.93g/g.With the increase of the
amount of crosslinker, the oil absorption rate of the modified material BMC gradually decreased.
4. Experimental results and discussion
Chemical modification of the bagasse cellulosic material was carried out with butyl methacrylate to
obtain a highly effective composite oil absorption material BMC.The optimal reaction conditions for
the preparation of BMC high-performance composites were as follows: reaction temperature 85 °C,
reaction time 4 h, raw material bagasse 3.00 g, graft monomer butyl methacrylate 2 ml, initiator
benzoyl peroxide.It is 0.1 g and the crosslinking agent methylenebisacrylamide is 0.01 g.
Acknowledgement
This research was financially supported by College Students' innovation training program of marine
technology and Environment College (2018), Dalian Ocean University postgraduate educational
reform project (2017)(02D0201 Lina Zheng)and Dalian Ocean University Students' innovation
and entrepreneurship program plan project(2017).
References
[1] Shen H Y 2016 Preparation and properties of biodegradable composites based on starch and
bagasse D. South China University of Technology
[2] Lao C, Yang X G, Li Y, Deng L G and Lu D J 2017Modification and Application of
Sugarcane Bagasse Cellulose J. Guangzhou Chemistry 42(02): 71-76.
[3] Zhou J M, Zou D, Lin G X, Niu X C and Wang J 2017Study on Adsorption Process of Cr(VI)
with Modified Bagasse J.Industrial Water and Wastewater 48(01):56-58.
[4] Su T C 2017 Preparation of carbon-based magnetic solid acid catalysts and their use in the
hydrolysis of plant wastes for sugar production D. University of Science and Technology
of China
[5] Zhu D Q, Sheng Y, He X P, Fan Y Y and Chen X 2017Effects of POE-g-(MAH/St) on the
properties of polypropylene/bagasse composites J. Journal of Fujian Normal
University(Natural Science) 33(04):47-51.
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
88
