
Stable Isotope Forensics for Predicting the Lifestyle and
Environmental Exposure of Unidentified Humans
E Dotsika
*
Stable Isotopes Unit, N.C.S.R. “Demokritos”, Institute of Nanoscience and
Nanotechnology, 15310, Ag.Paraskevi Attikis, Greece
Corresponding author and e-mail: E Dotsika, e.dotsika@inn.demokritos.gr
Abstract. In this study the relation of the
18
O isotope from meteoric waters and from teeth
enamel of samples from Crete was investigated.
13
C and
15
N isotopes measurements were
conducted on hair samples from Crete. The isotopic analysis was conducted in order to
determine diet and residence patterns.
1. Introduction
Isotopic fingerprint, in conjunction with the biological information from the skeleton, and the
epigenetic fingerprint can aid in the investigation of missing persons by identified the geographic
region from which a person is originating and can provide information on the lifestyle diet and socio-
economic status of unidentified humans. For this purpose, we conducted isotopic analysis of
13
C and
18
O of teeth enamel,
13
C and
15
N of hair and
18
O of spring water originating from the island of Crete
(Greece).
2. Sampling and methods
In this study water (spring and bottle waters) of Crete (Greece) were collected (15 and 2 samples
respectively)in order to provide the relation between
18
O of water and
18
O of teeth of known
human‟ssamples.15 teeth and hair samples from the Crete Island were analyzed in order to determine
the diet choices and residence pattern.
The isotopic composition of hydrogen (
2
H), oxygen (
18
O), carbon (
13
C) and Nitrogen (
15
N) was
measured in Stable Isotope Unit, Institute of Nanocience and Nanotechnology, NCSR Demokritos
(Athens, Greece) on a continuous flow Finnigan DELTA V plus (Thermo Electron Corporation,
Bremen, Germany) stable isotope mass spectrometer according to the procedures described in [1, 2].
The results are expressed in standard delta notation (δ) as per mil (‰) deviation from the standard
VSMOW as: δ= ((Rsample−Rstandard)/Rstandard)*1000 where Rsample and Rstandard =
2
H/
1
H or
18
O/
16
O or
13
C/
12
C ratios of sample and standard respectively. Measurement precision, based on the
repeated analysis of internal standard waters, was 1.5, 0.05, 0.5 and 0.5% for δ
2
H, δ
18
O, δ
13
C, and
δ
15
N respectively.
Dotsika, E.
Stable Isotope Forensics for Predicting the Lifestyle and Environmental Exposure of Unidentified Humans.
In Proceedings of the International Workshop on Environmental Management, Science and Engineering (IWEMSE 2018), pages 187-192
ISBN: 978-989-758-344-5
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
187

2.1 A subsection interpretation of isotope data from human remains
The C, N, H, O isotopic composition of human tissue will not match exactly that of the consumed
food and water. This is due to isotopic fractionation process: The differing kinetic and
thermodynamic properties of isotopes, due to biological, chemical and metabolic reactions, are
responsible for modifications in isotopic composition of the „light‟ bio-elements (carbon, hydrogen,
oxygen, sulfur).
Carbon isotope ratios of human tissues provide dietary information, specifically about the
photosynthetic pathway of plant material. Plants use three different photosynthetic pathways
important for human nutrition, characterized by distinctive isotope fractionations of carbon from CO
2
in the atmosphere to starch: C3, C4, and CAM (Crassulacean Acid Metabolism) which imparts
different
13
C/
12
C ratios to plant tissues. C3 plants (Calvin–Benson cycle: atmospheric CO
2
is fixed
through the reductive pentose phosphate pathway), include bushes, temperate shrubs and herbs, most
trees and domesticates such as wheat as well as grasses, that are favored by cool growing seasons
indicating cool/moist climate and/or high altitude. Modern C3 plants have an average d
13
C (VPDB)
value of -26 ± 5‰ versus PDB (Pee-Dee Belemnite, SC, USA) and typically range from -20‰ (open
areas exposed to water stress) to -35‰ (closed canopy). In the C4 plants (Hatch-Slack, C4-
dicarboxylic acid pathway) the
13
C values are on average about -13.0 ± 5‰ but generally range from
-9‰ to -19‰. C4 plants includes arid-adapted grasses and domesticates such as maize and sugar
cane, as well as a few desert shrubs and herbs and are common in tropical, subtropical and temperate
climates dominated by warm summer rainfall. The
13
C values of CAM plants (e.g., agave, pineapple)
range between the end members of C3 and C4 plants demonstrating an adaptation capacity in
keeping with their environmental conditions.
Carbon is ingested by human directly as vegetal and indirectly as animal products. The carbon
isotopic composition of tissues (like human hair or bone collagen) reflects the isotopic composition
of the diet with a slight offset of 1‰–3‰ [3, 4].
Nitrogen isotope ratios of animal tissues reflects the quality and quantity of protein consumed [5].
This is due to isotopic fractionation as nitrogen moves from lowest to highest trophic levels, resulting
in progressively higher δ
15
N values in animals relative to plants or animals lower on the food chain
[6]. The isotopic value into the tissues of the consumer present an increase of 3‰ per for each
trophic level. Carbon, nitrogen isotope ratio analyses are often used in determining whether or not an
individual has changed dietary habits.
The isotopic compositions of hydrogen and oxygen isotopes reflect natural processes in the
hydrological cycle. The isotopic ratio [(R =
2
H/
1
H or
18
O/
16
O; reported as δ
2
H or δ
18
O, where δ =
((RsampleRstandard)/Rstandard) 1000)] of fresh water varies greatly and systematically across the
earth as a result of the spatially and temporarily variable climatic patterns, which govern the delivery
of precipitated water to geographic regions. Strong trends in δ
2
H and δ
18
O occur with increases in
latitude, altitude, and continentality and these patterns are relatively well known and documented as
maps of precipitation stable isotope ratios [7-9]. So, locally the isotopic composition of precipitation
is primarily controlled by regional scale processes: it is greatly influenced by the provenance of wet
air masses, the trajectories of the water vapor transport over the continents, their possible partial
condensation in continental areas [10] and in general the average rainout history of the air masses
[11]. A rather complicated pattern has been observed in the Mediterranean basin, due to intense air-
sea interaction processes and the contribution of sea vapor to moisture-depleted continental air
masses. Warmer climates generally have higher δ
2
H and δ
18
O values of precipitation, while colder,
higher latitude locations have lower values. These spatial variations can be displayed graphically as
isotope landscapes, or isoscapes [12].
The local signals resulting from this predictable water isotope fractionation are propagated
through plants and animals and can be recovered from tissues (hair, tooth enamel, or bone) providing
geolocation information. The recovered signals will be characteristic of a range of isotopically
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
188
similar locations or iso-regions. When an individual moves from one iso-region to another the
modification of isotopic values of drinking water influences the isotopic composition of the body
water and are used to identify the residence patterns. Consequently geographic movements, for one
location to a new location, can be reconstructed by analyzing small segments along the length of hair
to provide a record of the last month or from tissues representing different periods of an individual‟s
life (tooth vs. bone vs. hair).
2.2 Results
The use of isotope analysis in modern forensic work would not be possible without of pioneering
work in the fields of geology, hydrogeology, anthropology, archaeology, ecology and plant
physiology [7-9]. Especially, the oxygen and hydrogen isotope analysis of water leads to construction
of the first isoscapes” maps, setting the base for the isotope forensics studies.
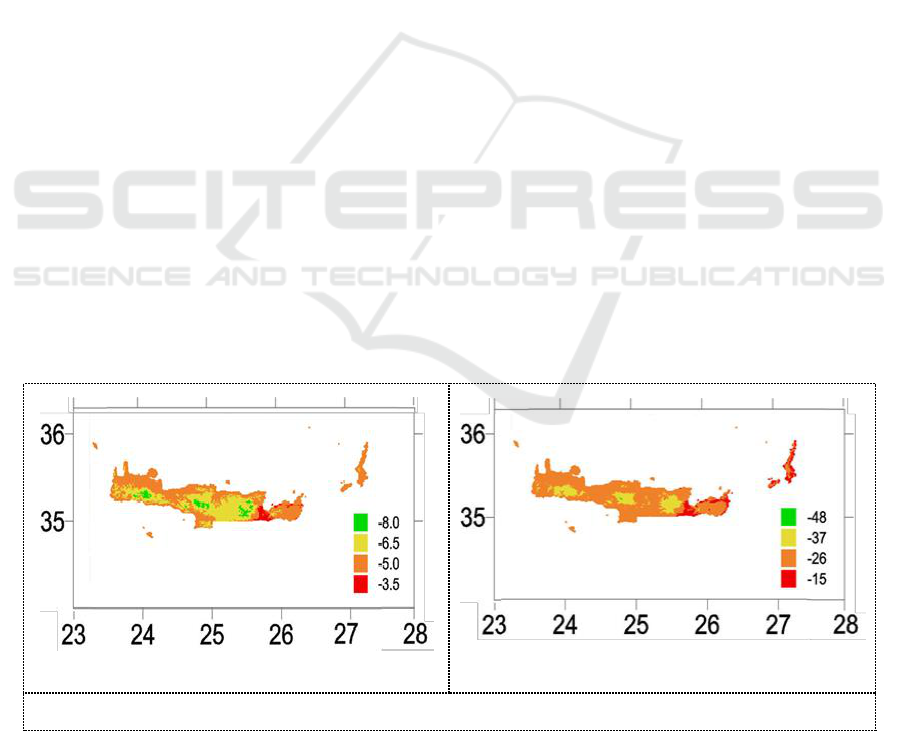
In this study, we present the oxygen isotope composition of spring waters from Crete aiming to
evaluate the spatial variability of spring water composition. The high resolution map of the spatial
distribution of spring water δ
18
O and δ
2
H (Figure 1) should provide important information for isotope
forensic studies. To obtain an overview of the spatial distribution of spring water δ
18
O, δ
2
H we
constructed gridded isotopic data sets with a resolution of 30′′ × 30′′ (approximately 1 km × 1 km)
using the methodology of Bowen and Wilkinson [13]. In order to achieve the highest possible
resolution we used the GTOPO30 data set maintained by the United States Geological Survey
(USGS, 2008). The oxygen and deuterium isotope values of spring waters range between −8.0‰ to
−3.5‰ and −48.0‰ to −15.0‰ respectively for Crete Island. The equation of Crete spring water
Local Meteoric Water Lines (LMWLCS) is given below and has a slope of 6:
δ
2
H= 6*δ
18
O +6.5
Generally, an isotope relationship between δ
2
H and δ
18
O with a slope of about 8 is normally
observed for precipitation [14] water. A slope of 6 is attributed to waters with a significant rate of
evaporation relative to input [14-16]. Also the weighted mean D-excess values, of 6.5 is not included
between the ranges from 10 for global precipitation to 22 for the eastern Mediterranean area [17, 18].
This decrease in LMWL slope and D-excess value in relation to the meteoric water (δ
2
H= 8.7*δ
18
O
+19.5, [7-9]) observed in spring waters across Crete confirms the evaporation of ground water.
Possible cause for this enrichment is the partial evaporation of water before the infiltration, the
infiltration of recycled irrigation water and evaporation of soil water.
(a)
(b)
Figure 1.Spatial distribution of spring water δ
18
O (a) and δ
2
H (b) of the island of Crete.
To predict potential origins from the δ
18
O value determined for the tooth enamel, we first
converted carbonate measurements to equivalent phosphate data [19] and then predicted drinking
water [20] from the phosphate data. In order to achieve that, several equations from international
Stable Isotope Forensics for Predicting the Lifestyle and Environmental Exposure of Unidentified Humans
189
literature may be used, which practically convert the δ
18
O of the carbonate component of the
bioapatite (δ
18
O
C
) to δ
18
O of the phosphate component (δ
18
O
P
) and finally to δ
18
O of water (δ
18
Ow).
As so, the δ
18
O
C
(vPDB) values need to be converted into δ
18
O
C
(vSMOW) using the established
equation:
δ
18
OvSMOW=1.03091* δ
18
O vPDB+30.91[21]
According to [19] the relationship between δ
18
O
P
and δ
18
O
C
values is expressed by the equation
δ
18
O
C
=1.015(±0.043)* δ
18
O
P
+8.79(±0.79), resulting through studies on bone and tooth samples of
modern mammals.
For the conversion of δ
18
O
P
in δ
18
O
w
Hoppe [20] suggested an equation that combines the structural
oxygen with the consuming water: δ
18
O
P
=21.28(±0.51)+[0.68(±0.04)*δ
18
O
w
].
The δ
18
O of enamel from the teeth samples of Crete ranges between -9.1‰ and -3.2‰. With the
exception of the most negative value (-9.1‰) the other isotopic data suggest that the individual drunk
tap water from the region of Crete where they passed all their lives. The most negative isotopic value
can be explained by the consumption of bottled waters from Crete (“Zaros” between -8.2‰ and -
7.9‰, and “Nera Critis” between -9.2‰ and 8.8‰ for the
18
O).
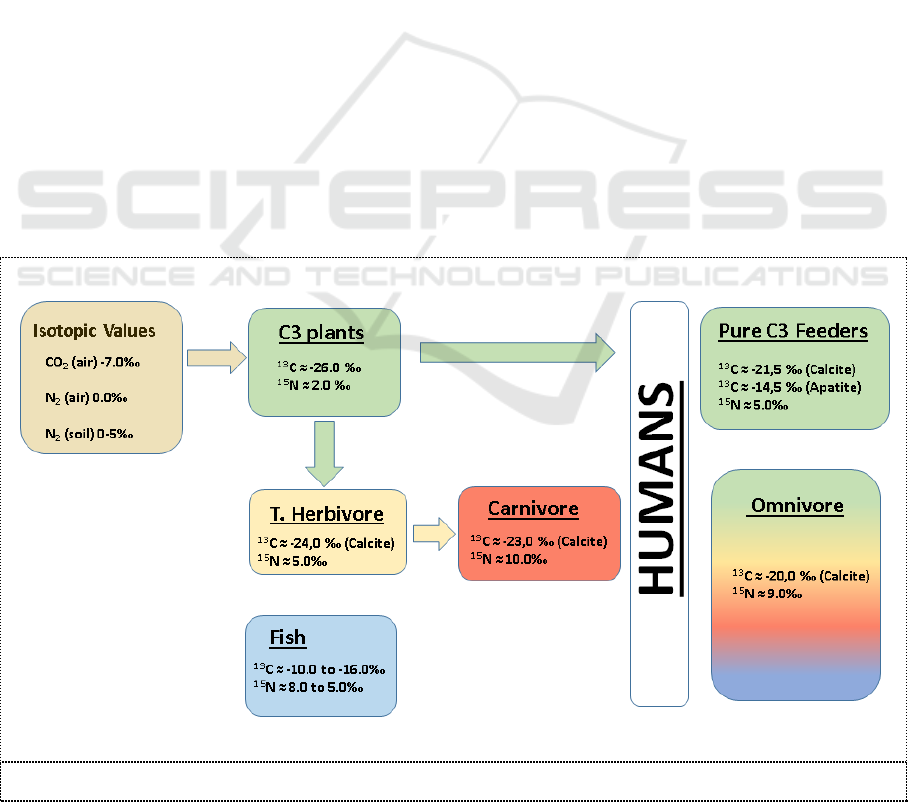
Isotopic values of
13
C of hair samples indicate the contribution of plant types to the diet. Also, the
knowledge of the specific plants makes it possible to interpret the contribution of different types of
animal protein to the diet, as animals that eat these plants will be isotopically similar [22]. Carbon
and Nitrogen isotopes can also be used in order to determine the contribution of terrestrial versus
marine proteins to the diet. All the above may aid in predicting region of origin or residence patterns
where cultural dietary patterns characterize a particular region like in our case the Mediterranian diet
[23, 24]. The measured values of
13
C range between -19‰ and -21.3‰ and for the
15
N between 8‰
and 8.6‰. These values from the hair samples originating from Crete are very similar to the
omnivore (Figure 2) values of humans but the lower
15
N value indicates that the percentage of
vegetable consumption in their diet is significant.
Figure 2. Typical Isotope values of the feeding chain.
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
190

3. Conclusions
The oxygen isotopic composition of the meteoric water is well correlated with the oxygen isotopic
composition of the teeth enamel in the teeth samples originating from the Island of Crete and
potentially can discriminate the provenance. The hair samples from the same area indicate omnivore
food consumption with significant contribution of vegetables that can be related to the Mediterranean
diet.
References
[1] Epstein S and T M 1953 Variation of 18O content of water from natural sources Geochim.
Cosmochim Acta 4: p 213–224
[2] Coleman M L and et al 1982 Reduction of water with zinc for hydrogen isotope analysis
Analytical chemistry 54(6): p 993-995
[3] Schoeninger M J and DeNiro M J 1984 Nitrogen and carbon isotopic composition of bone
collagen from marine and terrestrial animals Geochimica et Cosmochimica Acta 48(4): p
625-639
[4] Schoeninger M J 1985 Trophic level effects on 15N/14N and 13C/12C ratios in bone collagen
and strontium levels in bone mineral Journal of human evolution 14(5): p 515-525
[5] Robbins C T, Felicetti L A and Florin S T 2010 The impact of protein quality on stable
nitrogen isotope ratio discrimination and assimilated diet estimation Oecologia 162(3): p
571-579
[6] Post D M 2002 Using stable isotopes to estimate trophic position: models, methods, and
assumptions Ecology 83(3): p 703-718
[7] Dotsika E and et al 2010 Palaeo-climatic information from isotopic signatures of fossil teeth
in Late Pleistocene from Arkoudospilia Cave (Aridea, N. Greece) in EGU General
Assembly Conference Abstracts
[8] Michael D E and Dotsika E 2017 Using Oxygen Isotopic Values in Order to Infer
Palaeoclimatic Differences between Northern and Central-Southern Greece in IOP
Conference Series: Earth and Environmental Science IOP Publishing
[9] Michael D E and Dotsika E 2017 Using Oxygen and Carbon Isotopic Signatures in Order to
Infer Climatic and Dietary Information in Roman Edessa, Greece in IOP Conference
Series: Earth and Environmental Science IOP Publishing
[10] Merlivat L and Jouzel J 1979 Global climatic interpretation of the deuterium‐oxygen 18
relationship for precipitation Journal of Geophysical Research: Oceans 84(C8): p 5029-
5033
[11] Rozanski K, Sonntag C and Münnich K 1982 Factors controlling stable isotope composition of
European precipitation Tellus 34(2): p 142-150
[12] West J B and et al 2006 Stable isotopes as one of nature's ecological recorders Trends in
Ecology & Evolution 21(7): p 408-414
[13] Bowen G J and Wilkinson B 2002 Spatial distribution of δ18O in meteoric precipitation
Geology 30(4): p 315-318
[14] Craig H 1961 Isotopic variations in meteoric waters. Science 133(3465): p 1702-1703
[15] Craig H, Gordon L and Horibe Y 1963 Isotopic exchange effects in the evaporation of water:
1. Low‐temperature experimental results Journal of Geophysical Research 68(17): p
5079-5087
[16] Ehhalt D and et al 1963 Deuterium and oxygen 18 in rain water Journal of Geophysical
Research 68(13): p 3775-3780
[17] Gat J and Carmi I 1970 Evolution of the isotopic composition of atmospheric waters in the
Mediterranean Sea area Journal of Geophysical Research 75(15): p 3039-3048
[18] Nir A 1967 Development of isotope methods applied to groundwater hydrology Isotope
Stable Isotope Forensics for Predicting the Lifestyle and Environmental Exposure of Unidentified Humans
191

Techniques in the Hydrologic Cycle p 109-116
[19] Iacumin P and et al 1996 Oxygen isotope analyses of co-existing carbonate and phosphate in
biogenic apatite: a way to monitor diagenetic alteration of bone phosphate? Earth and
Planetary Science Letters 142(1-2): p. 1-6
[20] Hoppe K A 2006 Correlation between the oxygen isotope ratio of North American bison teeth
and local waters: implication for paleoclimatic reconstructions Earth and Planetary Science
Letters 244(1-2): p 408-417
[1] Coplen T B and Kendall C and Hopple J 1983 Comparison of stable isotope reference samples
Nature 302(5905): p 236
[21] DeNiro M J and Epstein S 1978 Influence of diet on the distribution of carbon isotopes in
animals Geochimica et cosmochimica acta 42(5): p 495-506
[22] Bartelink E, Berry R and Chesson L 2014 Stable isotopes and human provenancing Advances
in forensic human identification p 165-192
[23] Lehn C, Rossmann A and Graw M 2015 Provenancing of unidentified corpses by stable
isotope techniques–presentation of case studies Science and Justice 55(1): p 72-88
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
192
