
Determination of the Mercury Solubility in Several Natural
Gas Processing Fluids
Q T Yan
1,*
, Y L Zhao
2
, J Li
1
, Y F Duan
2
, S Y Wang
1
, X Z Geng
2
and J L Ma
3
1
Petrochina Research Institute of Petrolum Exploration and Development, Langfang
065007, China
2
School of Energy and Environment, Southeast University, Nanjing 210096, China
3
Daqing Oilfield Gas Production Company, Daqing 163000, China
Corresponding author and e-mail: Q T Yan, yanqituan69@petrochina.com.cn
Abstract. The high concentration of mercury present in natural gas and the processing fluids
can cause severe problems such as corrosion of installations, reduced catalyst life and
environment pollution. The determination of mercury solubility in the processing fluids is
required to determine the migration and distribution of mercury in various logistics during the
production process and improve the efficiency of mercury removal. For this purpose, the
solubility and the dissolving characteristics of mercury in several natural gas processing
fluids (MEG, TEG and MDEA in particular) were studied at a temperature range from 253K
to 373K. The dissolution experiments were carried out in the dissolving kettle and mercury
concentration was analyzed by cold-vapor atomic absorption technique (CVAAS). The
experimental results indicated that the solubility of mercury in these solvents showed an
increasing trend with increased temperature and pressure. The results would be useful for the
mercury pollution control and environmental risk management.
1. Introduction
With the increasing concern of mercury pollution, the mercury emission from the production and
processing industries of natural gas has gradually been paid more attention from all over the world,
and many countries are drawing up relevant measures and policies for the prevention and control of
mercury [1-2]. Due to the high dispersion and volatility in the lithosphere, mercury is widely
distributed in natural gas reservoirs [3]. Researches have shown that the limit of mercury
concentration in natural gas is no higher than 30 μg/m
3
, which will not cause harm to equipment,
personal safety and environment [4].
In the process of dehydration, de-hydrocarbon and de-acidification, mercury in the feed gas can be
adsorbed by the pipe wall, discharged into the atmosphere with flash gas and regenerative gas, or
drained into the sewage pool through sewage sludge, etc. Through on-site sampling and analysis, the
concentration of mercury in this series of purification processes continuously decreases [5-6].
Therefore, it is of great interest to study the solubility and the dissolving characteristics of mercury in
the natural gas processing fluids, especially in the MEG, TEG, and MDEA, which are widely used in
the process of natural gas treatment.
Yan, Q., Zhao, Y., Li, J., Duan, Y., Wang, S., Geng, X. and Ma, J.
Determination of the Mercury Solubility in Several Natural Gas Processing Fluids.
In Proceedings of the International Workshop on Environmental Management, Science and Engineering (IWEMSE 2018), pages 201-206
ISBN: 978-989-758-344-5
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
201
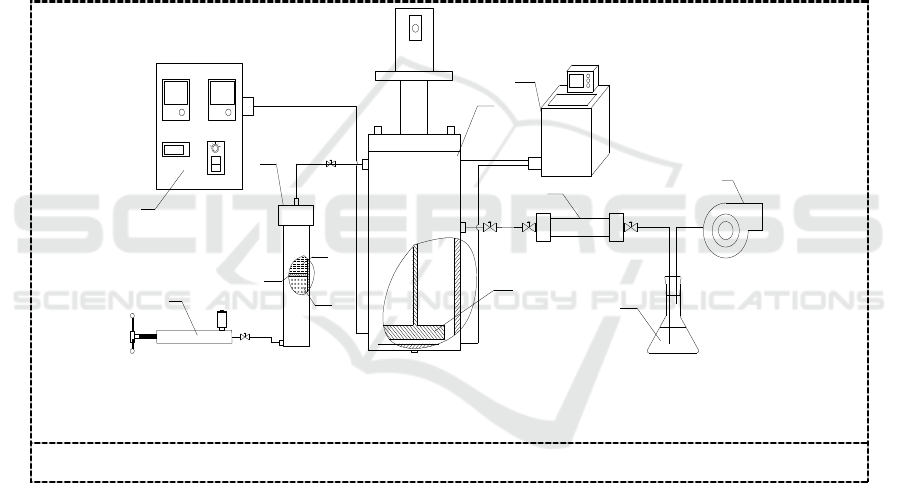
In this paper, a set of experimental device for determination of mercury solubility was established.
The solubility and the dissolving characteristics of mercury in the MEG, TEG, MDEA and water
were studied at a temperature range from 253K to 373K. In addition, pressure dissolution
experiments were carried out. The experimental data will help to determine the migration and
distribution of mercury in various logistics during the production process.
2. Methodology
2.1. Experimental apparatus and procedure
Due to the low dissolution rate and volatility of mercury, the solvents were very easy to evaporate
when dissolving and sampling, especially in the high temperature experiments, which may cause
mercury loss [7]. Therefore, a new method was developed and a set of solubility measurement
devices was designed, which was mainly composed of temperature and pressure control system,
magnetic stirring system, dissolving kettle and sampling system. The diagram of the apparatus is
shown in Figure 1.
1
2
3
3b
3a
3c
4a
5
6
8
7
T
ON
P
OFF
n
4
1. pressuring pump ; 2.control panel ; 3. charging stock tank (3a piston; 3b solvents; 3c water);
4.dissolving kettle (4a stirring rake); 5.temperature control system ; 6.sampling tube ; 7.absorption
bottle ; 8. vacuum pump
Figure 1. Experimental apparatus for solubility determination.
The experiments were performed by placing 800ml of solvent spiked with 10g of liquid mercury
(as Hg
0
) into the dissolving kettle. All the solvents were stirred together at a given temperature and
pressure for about 6 h, then the upper solution was analyzed after the stirring stopped for about 2 h,
and the concentration of mercury was considered as the solubility at the temperature. The sampling
tube was vacuumed by means of the pump before the sampling operation to avoid mercury
evaporation loss. In order to eliminate the influence of temperature fluctuation, the samples were
quickly transferred into digestion tubes in water bath for further digestion and analysis.
2.2. Analytical method
The generally accepted procedure for the determination of trace amounts of mercury is the cold-
vapor atomic absorption technique (CVAAS) because of its high sensitivity, which consists of
stannous chloride reduction, nitrogen bubbling, and passing through a magnesium-perchlorate tube
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
202
for mercury-vapor drying [8]. The mercury concentration was determined from the area under the
atomic absorption peak at 253.7 nm.
In this study, the sample digestion was carried out by the wet closed digestion combined with
water bath and hydrogen peroxide mixed with nitric acid was used for digestion. The optimum ratio
was determined as HNO
3
: H
2
O
2
= 5:1 through a number of preliminary experiments. After digested
and cooled to room temperature, the sample was diluted with 5% HNO
3
, and then analysed by
automatic mercury measurement instrument Hydra AA (Leeman Labs Inc., USA). The analysis of all
samples were done in triplicate, and the relative standard deviation (RSD) of the 3 replicates was
controlled less than 3%. The minimum detection limit of the instrument was 1ng/L.
Calculation formula of solubility (equilibrium concentration) was described as follow:
Where X was the solubility of mercury in the sample, c was the concentration of mercury in the
sample after digestion, V was the total volume of the sample after digestion and m was the weight of
sample. The unit of mercury solubility is ng/g and usually expressed as ppb.
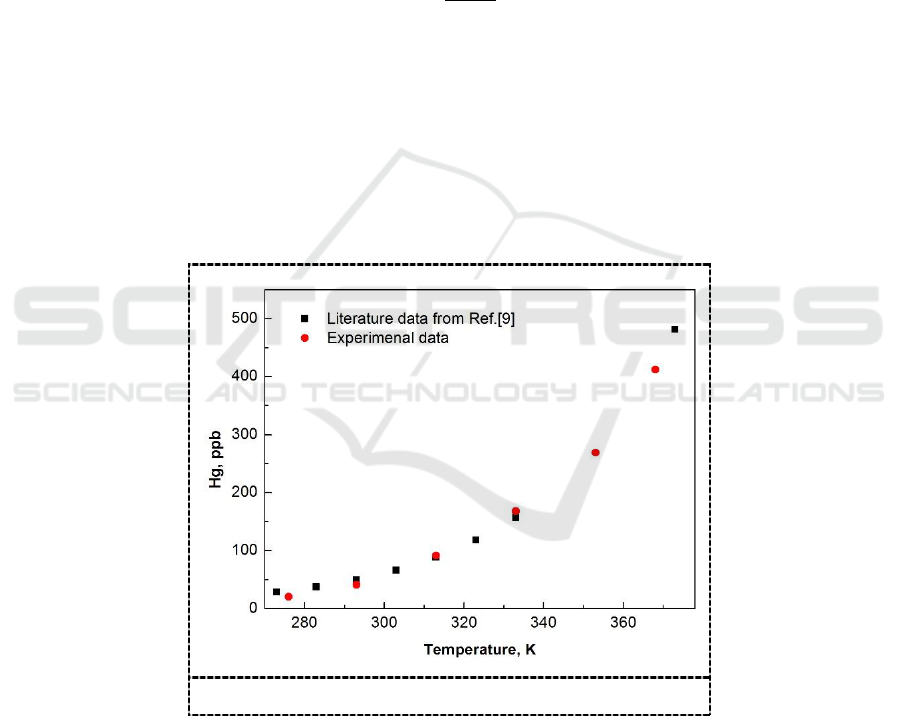
To verify the reliability of the experimental apparatus, solubility of mercury in water was
compared with literature data [9]. The result is shown in Figure 2. It can be seen that the
experimental data reported in this study are in agreement with the data from the literature, and the
biggest relative deviation calculated between the solubility of the literature and the measured
solubility of this study is less than 5%.
Figure 2. Solubility of mercury in water.
3. Results and discussion
3.1. Mercury solubility determination
The measured solubility data of mercury in the studied solvents within their respective operating
temperature ranges are shown in Figure 3. The results indicated that the solubility of liquid mercury
in MDEA, MEG and TEG shows a temperature dependence and is much higher than that in water,
which may result from the hydroxyl groups in these solvent molecules and interaction between
mercury and solvent molecules. The maximum solubility of mercury was reported in TEG (99%) and
Determination of the Mercury Solubility in Several Natural Gas Processing Fluids
203
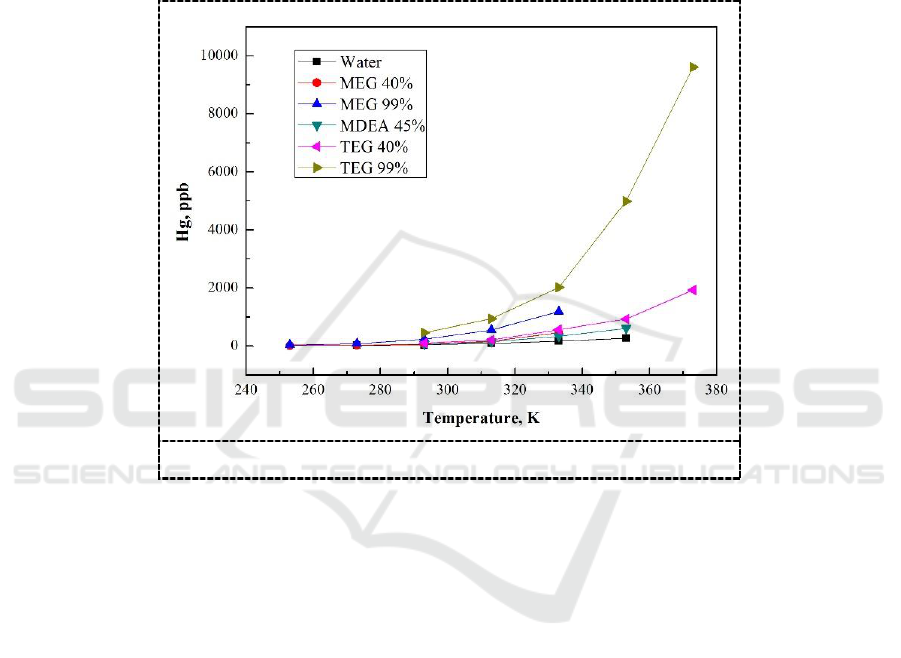
was 9610 ppb at 373K followed by that in MEG (99%, 1185 ppb, at 333K) and MDEA (45%, 614
ppb, at 353K). The solubility of mercury in TEG is only about one-tenth of the solubility in an alkane,
but it is almost 30 times more soluble than in water [10].
In addition, dielectric constant of the solvents is in the following order: Water > Glycols > Amine
[11]. Comparison of solubility data as a function of dielectric constant of solvents suggests that the
solubility of mercury increases with a decrease in the dielectric constant of amine and glycols. The
measured solubility values of pure glycols in this study were found in a good agreement with the
literature values [9].
Figure 3. Solubility of mercury in the studied solvents.
The main potential source of error in the experimental method employed in this work is the loss of
mercury due to adsorption of Hg on the walls of the sampling container. This error is considered to
be negligible, because the measured concentration of mercury in repeated experiments was
reproducible to 98%.
The discrepancy of solubility data in the literature available may be due to the different digestion
methods and the different detection instruments. Because the mercury concentration in these solvents
were at ppb grade, and the experimental conditions and analytical methods have a great impact on the
experiment results. In this study, the dissolution process occurs in a closed reactor to ensure that the
dissolution process does not contact with the atmosphere, thus avoiding the interference of
environmental oxidation.
3.2. The effect of pressure on mercury solubility
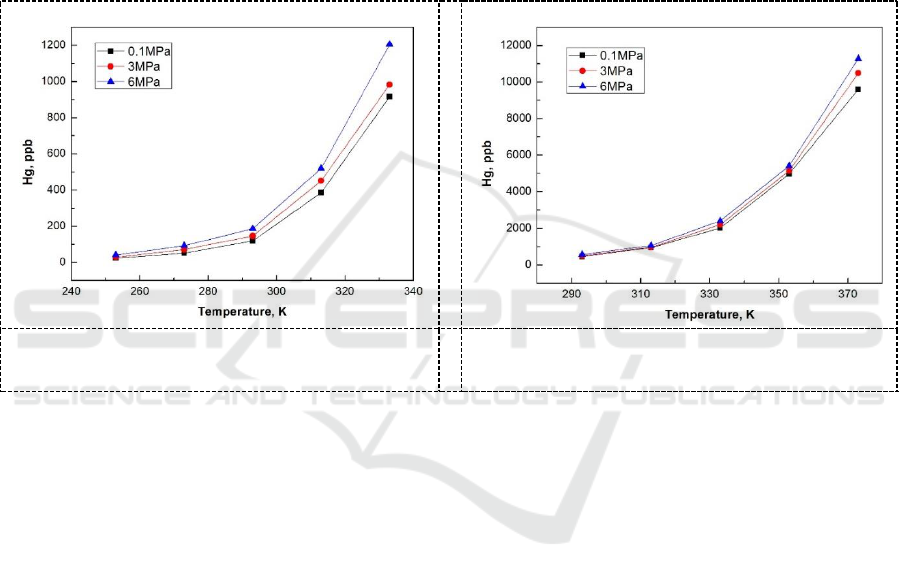
Through pressure dissolution experiments, it was found that with the increase of pressure, the
equilibrium concentration of mercury in MEG and TEG increased slightly at the same temperature,
but the effect of pressure on the solubility of mercury was not obvious compared with the
temperature. The variation of solubility in MEG and TEG with pressure is shown in Figure 4 and
Figure 5.
According to M M Miedaner et al. [12], the dissolution of metallic mercury into the polar solvent
can be expressed by the following reactions:
Hg
0
↔ Hg
(gas)
(1)
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
204
Hg
(gas)
↔ Hg
(diss)
(2)
which can be simplified to an overall reaction:
Hg
0
↔ Hg
(diss)
(3)
The effect of pressure on the dissolution of liquid is quite complex. Volatile liquids have the
properties similar to the gas, and their solubility may be greatly influenced by pressure [13].
Therefore, it may be speculated that the phenomenon that the solubility of mercury increases with the
increase of pressure may be due to the volatility of the mercury that makes it similar to the solubility
of gas. According to the law of gas dissolution, the pressure increases and the solubility of gas
increases.
Figure 4. The effect of pressure on mercury
solubility in MEG.
Figure 5. The effect of pressure on mercury
solubility in TEG.
3.3. Application of the solubility data
According to the experimental data of mercury solubility in this study, a large amount of mercury in
natural gas may enter the rich gas processing solvents. Although there is no limit of the mercury
concentration in the glycol and amine quality index, sewage discharge from the regeneration process
of gas processing solvents should be carried out by GB8978-1996 Integrated Wastewater Discharge
Standard of the state, and the maximum permitted discharge concentration of total mercury is
0.05mg/L. The government of Thailand requires that the total mercury concentration of the treated
sewage is less than 0.005 mg/L, and the mercury emission limits of the United States are as low as
0.079 μg/L [14].
The enrichment of mercury in natural gas treatment equipment may be harmful to the health of
operators and maintenance personnel. In particular, when mercury-contaminated facilities are
repaired during maintenance, the staff will be exposed to high concentration of mercury vapor above
the valve limit and maximum allowable concentration if no special precautions are taken.
4. Conclusions
The solubility of liquid elemental mercury in the natural gas processing solvents (MEG, TEG and
MDEA) was determined within their respective operating temperature ranges. Experimental results
show that there are great differences in the solubility of mercury in different solvents. The solubility
of mercury in these solvents increased with temperature ranging from 253K-373K. In particular,
mercury solubility in the solvents at range of 273K-353K follows the general order: Glycols (TEG,
Determination of the Mercury Solubility in Several Natural Gas Processing Fluids
205

MEG) > MDEA > Water. In addition, the pressure dissolving experiment indicated that mercury
solubility in MEG and TEG increased slightly with the increased pressure.
References
[1] Ryzhov V V, Mashyanov N R and Ozerova N A 2003 J. Science of the Total Environment
304(1) 145-152
[2] Wilhelm S M, Bloom N 2000 J. Fuel Processing Technology 63(1) 1-27
[3] Clever H L 1987 Mercury in liquids, compressed gases, molten salts and other elements ed H
L Clever (London: Pergamon Press) pp 150-173
[4] Okouchi S and Sasaki S 2006 J. Bull.chem.soc.jpn 54(8) 2513-2514
[5] Gallup D L, O'Rear D J and Radford R 2017 J. Fuel 196 178-184
[6] Gajdosechova Z, Boskamp M S and Lopezlinares F 2016 J. Energy & Fuels 30(1) 130-137
[7] Frech W, Baxter D C and Dyvik G 1995 J. Journal of Analytical Atomic Spectrometry 10(10)
769-775
[8] Clever H L, Iwamoto M 1987 J. Industrial & Engineering Chemistry Research 26(2) 336-337
[9] Akerlof G 1932 J. Journal of the American Chemical Society 54(11) 4125-4139
[10] Benoit J M, Mason R P and Gilmour C C 2001 J. Geochimica Et Cosmochimica Acta 65(24)
4445-4451
[11] Ravichandran M 2004 J. Chemosphere 55(3) 319-331
[12] Miedaner M M, Migdisov A A and A E Williams-Jones 2005 J. Geochimica Et Cosmochimica
Acta 69(23) 5511-5516
[13] Polishuk I, Vilk A and Chorazewski M 2017 J. Fuel 203 686–689
[14] Marsh K N, Bevan J W and Holste J C 2016 J. Journal of Chemical & Engineering Data
61(8) 2805-2817
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
206
